Cocaine
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | kə(ʊ)ˈkeɪn |
| Trade names | Neurocaine,[1] Goprelto,[2] Numbrino,[3] others |
| Other names | Coke, blow, snow, yay, crack (in free base form) |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| License data | |
| Dependence liability | Physical: Low Psychological: High[4] |
| Addiction liability | High[5] |
| Routes of administration | Topical, by mouth, insufflation, intravenous, inhalation |
| Drug class | |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability |
|
| Metabolism | Liver, CYP3A4 |
| Metabolites | Norcocaine, benzoylecgonine, cocaethylene |
| Onset of action | Seconds to minutes[12] |
| Duration of action | 20 to 90 minutes[12] |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.030 |
| Chemical and physical data | |
| Formula | C17H21NO4 |
| Molar mass | 303.358 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 98 °C (208 °F) |
| Boiling point | 187 °C (369 °F) |
| Solubility in water | 1.8g/L (22 °C) |
| |
| |
| Data page | |
| Cocaine (data page) | |
| | |
Cocaine (from French: cocaïne, from Spanish: coca, ultimately from Quechua: kúka)[13] is a tropane alkaloid that acts as a central nervous system (CNS) stimulant. As an extract, it is mainly used recreationally and often illegally for its euphoric and rewarding effects. It is also used in medicine by Indigenous South Americans for various purposes and rarely, but more formally, as a local anaesthetic or diagnostic tool by medical practitioners in more developed countries. It is primarily obtained from the leaves of two Coca species native to South America: Erythroxylum coca and E. novogranatense.[14][15] After extraction from the plant, and further processing into cocaine hydrochloride (powdered cocaine), the drug is administered by being either snorted, applied topically to the mouth, or dissolved and injected into a vein. It can also then be turned into free base form (typically crack cocaine), in which it can be heated until sublimated and then the vapours can be inhaled.[12]
Cocaine stimulates the mesolimbic pathway in the brain.[15] Mental effects may include an intense feeling of happiness, sexual arousal, loss of contact with reality, or agitation.[12] Physical effects may include a fast heart rate, sweating, and dilated pupils.[12] High doses can result in high blood pressure or high body temperature.[16] Onset of effects can begin within seconds to minutes of use, depending on method of delivery, and can last between five and ninety minutes.[12] As cocaine also has numbing and blood vessel constriction properties, it is occasionally used during surgery on the throat or inside of the nose to control pain, bleeding, and vocal cord spasm.[17]
Cocaine crosses the blood–brain barrier via a proton-coupled organic cation antiporter[18][19] and (to a lesser extent) via passive diffusion across cell membranes.[20] Cocaine blocks the dopamine transporter,[21] inhibiting reuptake of dopamine from the synaptic cleft into the pre-synaptic axon terminal; the higher dopamine levels in the synaptic cleft increase dopamine receptor activation in the post-synaptic neuron,[22][23] causing euphoria and arousal.[24] Cocaine also blocks the serotonin transporter and norepinephrine transporter, inhibiting reuptake of serotonin and norepinephrine from the synaptic cleft into the pre-synaptic axon terminal and increasing activation of serotonin receptors and norepinephrine receptors in the post-synaptic neuron, contributing to the mental and physical effects of cocaine exposure.[6]
A single dose of cocaine induces tolerance to the drug's effects.[25] Repeated use is likely to result in addiction. Addicts who abstain from cocaine may experience prolonged craving lasting for many months.[26][27] Abstaining addicts also experience modest drug withdrawal symptoms lasting up to 24 hours, with sleep disruption, anxiety, irritability, crashing, depression, decreased libido, decreased ability to feel pleasure, and fatigue being common.[28][15] Use of cocaine increases the overall risk of death, and intravenous use potentially increases the risk of trauma and infectious diseases such as blood infections and HIV through the use of shared paraphernalia. It also increases risk of stroke, heart attack, cardiac arrhythmia, lung injury (when smoked), and sudden cardiac death.[15][29] Illicitly sold cocaine can be adulterated with fentanyl, local anesthetics, levamisole, cornstarch, quinine, or sugar, which can result in additional toxicity.[30][31] In 2017, the Global Burden of Disease study found that cocaine use caused around 7,300 deaths annually.[32]
Uses
Coca leaves have been used by Andean civilizations since ancient times.[30] In ancient Wari culture,[33] Inca culture, and through modern successor indigenous cultures of the Andes mountains, coca leaves are chewed, taken orally in the form of a tea, or alternatively, prepared in a sachet wrapped around alkaline burnt ashes, and held in the mouth against the inner cheek; it has traditionally been used to combat the effects of cold, hunger, and altitude sickness.[34][35] Cocaine was first isolated from the leaves in 1860.[15]
Globally, in 2019, cocaine was used by an estimated 20 million people (0.4% of adults aged 15 to 64 years). The highest prevalence of cocaine use was in Australia and New Zealand (2.1%), followed by North America (2.1%), Western and Central Europe (1.4%), and South and Central America (1.0%).[36] Since 1961, the Single Convention on Narcotic Drugs has required countries to make recreational use of cocaine a crime.[37] In the United States, cocaine is regulated as a Schedule II drug under the Controlled Substances Act, meaning that it has a high potential for abuse but has an accepted medical use.[38] While rarely used medically today, its accepted uses are as a topical local anesthetic for the upper respiratory tract as well as to reduce bleeding in the mouth, throat and nasal cavities.[39]
Medical
Cocaine eye drops are frequently used by neurologists when examining patients suspected of having Horner syndrome. In Horner syndrome, sympathetic innervation to the eye is blocked. In a healthy eye, cocaine will stimulate the sympathetic nerves by inhibiting norepinephrine reuptake, and the pupil will dilate; if the patient has Horner syndrome, the sympathetic nerves are blocked, and the affected eye will remain constricted or dilate to a lesser extent than the opposing (unaffected) eye which also receives the eye drop test. If both eyes dilate equally, the patient does not have Horner syndrome.[40]

Topical cocaine is sometimes used as a local numbing agent and vasoconstrictor to help control pain and bleeding with surgery of the nose, mouth, throat or lacrimal duct. Although some absorption and systemic effects may occur, the use of cocaine as a topical anesthetic and vasoconstrictor is generally safe, rarely causing cardiovascular toxicity, glaucoma, and pupil dilation.[41][42] Occasionally, cocaine is mixed with adrenaline and sodium bicarbonate and used topically for surgery, a formulation called Moffett's solution.[43]
Cocaine hydrochloride (Goprelto), an ester local anesthetic, was approved for medical use in the United States in December 2017, and is indicated for the introduction of local anesthesia of the mucous membranes for diagnostic procedures and surgeries on or through the nasal cavities of adults.[44][2] Cocaine hydrochloride (Numbrino) was approved for medical use in the United States in January 2020.[45][3]
The most common adverse reactions in people treated with Goprelto are headache and epistaxis.[2] The most common adverse reactions in people treated with Numbrino are hypertension, tachycardia, and sinus tachycardia.[3]
Recreational
Cocaine is a central nervous system stimulant.[46] Its effects can last from 15 minutes to an hour. The duration of cocaine's effects depends on the amount taken and the route of administration.[47] Cocaine can be in the form of fine white powder and has a bitter taste. Crack cocaine is a smokeable form of cocaine made into small "rocks" by processing cocaine with sodium bicarbonate (baking soda) and water.[12][48] Crack cocaine is referred to as "crack" because of the crackling sounds it makes when heated.[12]
Cocaine use leads to increases in alertness, feelings of well-being and euphoria, increased energy and motor activity, and increased feelings of competence and sexuality.[49]
Analysis of the correlation between the use of 18 various psychoactive substances shows that cocaine use correlates with other "party drugs" (such as ecstasy or amphetamines), as well as with heroin and benzodiazepines use, and can be considered as a bridge between the use of different groups of drugs.[50]
Coca leaves
It is legal for people to use coca leaves in some Andean nations, such as Peru and Bolivia, where they are chewed, consumed in the form of tea, or are sometimes incorporated into food products.[51] Coca leaves are typically mixed with an alkaline substance (such as lime) and chewed into a wad that is retained in the buccal pouch (mouth between gum and cheek, much the same as chewing tobacco is chewed) and sucked of its juices. The juices are absorbed slowly by the mucous membrane of the inner cheek and by the gastrointestinal tract when swallowed. Alternatively, coca leaves can be infused in liquid and consumed like tea. Coca tea, an infusion of coca leaves, is also a traditional method of consumption. The tea has often been recommended for travelers in the Andes to prevent altitude sickness.[52] Its actual effectiveness has never been systematically studied.[52]
In 1986 an article in the Journal of the American Medical Association revealed that U.S. health food stores were selling dried coca leaves to be prepared as an infusion as "Health Inca Tea". While the packaging claimed it had been "decocainized", no such process had actually taken place. The article stated that drinking two cups of the tea per day gave a mild stimulation, increased heart rate, and mood elevation, and the tea was essentially harmless.[53]
Insufflation

Nasal insufflation (known colloquially as "snorting", "sniffing", or "blowing") is a common method of ingestion of recreational powdered cocaine.[54] The drug coats and is absorbed through the mucous membranes lining the nasal passages. Cocaine's desired euphoric effects are delayed when snorted through the nose by about five minutes. This occurs because cocaine's absorption is slowed by its constricting effect on the blood vessels of the nose.[12] Insufflation of cocaine also leads to the longest duration of its effects (60–90 minutes).[12] When insufflating cocaine, absorption through the nasal membranes is approximately 30–60%[55]
In a study of cocaine users, the average time taken to reach peak subjective effects was 14.6 minutes.[56] Any damage to the inside of the nose is due to cocaine constricting blood vessels — and therefore restricting blood and oxygen/nutrient flow — to that area.
Rolled up banknotes, hollowed-out pens, cut straws, pointed ends of keys, specialized spoons,[57] long fingernails, and (clean) tampon applicators are often used to insufflate cocaine. The cocaine typically is poured onto a flat, hard surface (such as a mobile phone screen, mirror, CD case or book) and divided into "bumps", "lines" or "rails", and then insufflated.[58] A 2001 study reported that the sharing of straws used to "snort" cocaine can spread blood diseases such as hepatitis C.[59]
Injection
Subjective effects not commonly shared with other methods of administration include a ringing in the ears moments after injection (usually when over 120 milligrams) lasting 2 to 5 minutes including tinnitus and audio distortion. This is colloquially referred to as a "bell ringer". In a study of cocaine users, the average time taken to reach peak subjective effects was 3.1 minutes.[56] The euphoria passes quickly. Aside from the toxic effects of cocaine, there is also the danger of circulatory emboli from the insoluble substances that may be used to cut the drug. As with all injected illicit substances, there is a risk of the user contracting blood-borne infections if sterile injecting equipment is not available or used.
An injected mixture of cocaine and heroin, known as "speedball", is a particularly dangerous combination, as the converse effects of the drugs actually complement each other, but may also mask the symptoms of an overdose. It has been responsible for numerous deaths, including celebrities such as comedians/actors John Belushi and Chris Farley, Mitch Hedberg, River Phoenix, grunge singer Layne Staley and actor Philip Seymour Hoffman. Experimentally, cocaine injections can be delivered to animals such as fruit flies to study the mechanisms of cocaine addiction.[60]
Inhalation
The onset of cocaine's euphoric effects is fastest with inhalation, beginning after 3–5 seconds.[12] This gives the briefest euphoria (5–15 minutes).[12] Cocaine is smoked by inhaling the vapor produced when crack cocaine is heated to the point of sublimation.[61] In a 2000 Brookhaven National Laboratory medical department study, based on self-reports of 32 people who used cocaine who participated in the study, "peak high" was found at a mean of 1.4 ± 0.5 minutes.[56] Pyrolysis products of cocaine that occur only when heated/smoked have been shown to change the effect profile, i.e. anhydroecgonine methyl ester, when co-administered with cocaine, increases the dopamine in CPu and NAc brain regions, and has M1 — and M3 — receptor affinity.[62]
People often freebase crack with a pipe made from a small glass tube, often taken from "love roses", small glass tubes with a paper rose that are promoted as romantic gifts.[63] These are sometimes called "stems", "horns", "blasters" and "straight shooters". A small piece of clean heavy copper or occasionally stainless steel scouring pad – often called a "brillo" (actual Brillo Pads contain soap, and are not used) or "chore" (named for Chore Boy brand copper scouring pads) – serves as a reduction base and flow modulator in which the "rock" can be melted and boiled to vapor. Crack is smoked by placing it at the end of the pipe; a flame held close to it produces vapor, which is then inhaled by the smoker. The effects felt almost immediately after smoking, are very intense and do not last long — usually 2 to 10 minutes.[64] When smoked, cocaine is sometimes combined with other drugs, such as cannabis, often rolled into a joint or blunt.
Effects
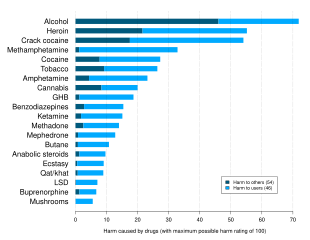
-
Opioid involvement in cocaine overdose deaths in the US. The pale green line is cocaine without any opioid (bottom line in 2017). The yellow line is cocaine and synthetic opioids other than methadone (top line in 2017).[66]
-
Delphic analysis regarding 20 popular recreational drugs based on expert opinion in the UK. Cocaine was ranked the 2nd in dependence and physical harm and 3rd in social harm.[67]
Acute
Acute exposure to cocaine has many effects on humans, including euphoria, increases in heart rate and blood pressure, and increases in cortisol secretion from the adrenal gland.[68] In humans with acute exposure followed by continuous exposure to cocaine at a constant blood concentration, the acute tolerance to the chronotropic cardiac effects of cocaine begins after about 10 minutes, while acute tolerance to the euphoric effects of cocaine begins after about one hour.[25][69][70][71] With excessive or prolonged use, the drug can cause itching, fast heart rate, and paranoid delusions or sensations of insects crawling on the skin.[72] Intranasal cocaine and crack use are both associated with pharmacological violence. Aggressive behavior may be displayed by both addicts and casual users. Cocaine can induce psychosis characterized by paranoia, impaired reality testing, hallucinations, irritability, and physical aggression. Cocaine intoxication can cause hyperawareness, hypervigilance, and psychomotor agitation and delirium. Consumption of large doses of cocaine can cause violent outbursts, especially by those with preexisting psychosis. Crack-related violence is also systemic, relating to disputes between crack dealers and users.[73] Acute exposure may induce cardiac arrhythmias, including atrial fibrillation, supraventricular tachycardia, ventricular tachycardia, and ventricular fibrillation. Acute exposure may also lead to angina, heart attack, and congestive heart failure.[74] Cocaine overdose may cause seizures, abnormally high body temperature and a marked elevation of blood pressure, which can be life-threatening,[72] abnormal heart rhythms,[75] and death.[75] Anxiety, paranoia, and restlessness can also occur, especially during the comedown. With excessive dosage, tremors, convulsions and increased body temperature are observed.[46] Severe cardiac adverse events, particularly sudden cardiac death, become a serious risk at high doses due to cocaine's blocking effect on cardiac sodium channels.[75] Incidental exposure of the eye to sublimated cocaine while smoking crack cocaine can cause serious injury to the cornea and long-term loss of visual acuity.[76]
Chronic
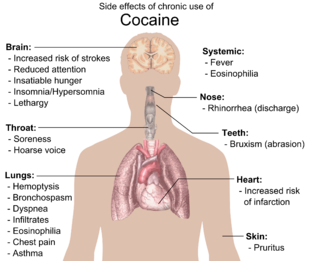
Although it has been commonly asserted, the available evidence does not show that chronic use of cocaine is associated with broad cognitive deficits.[77] Research is inconclusive on age-related loss of striatal dopamine transporter (DAT) sites, suggesting cocaine has neuroprotective or neurodegenerative properties for dopamine neurons.[78][79][80] Exposure to cocaine may lead to the breakdown of the blood–brain barrier.[81][82]
Physical side effects from chronic smoking of cocaine include coughing up blood, bronchospasm, itching, fever, diffuse alveolar infiltrates without effusions, pulmonary and systemic eosinophilia, chest pain, lung trauma, sore throat, asthma, hoarse voice, dyspnea (shortness of breath), and an aching, flu-like syndrome. Cocaine constricts blood vessels, dilates pupils, and increases body temperature, heart rate, and blood pressure. It can also cause headaches and gastrointestinal complications such as abdominal pain and nausea. A common but untrue belief is that the smoking of cocaine chemically breaks down tooth enamel and causes tooth decay. Cocaine can cause involuntary tooth grinding, known as bruxism, which can deteriorate tooth enamel and lead to gingivitis.[83] Additionally, stimulants like cocaine, methamphetamine, and even caffeine cause dehydration and dry mouth. Since saliva is an important mechanism in maintaining one's oral pH level, people who use cocaine over a long period of time who do not hydrate sufficiently may experience demineralization of their teeth due to the pH of the tooth surface dropping too low (below 5.5). Cocaine use also promotes the formation of blood clots.[12] This increase in blood clot formation is attributed to cocaine-associated increases in the activity of plasminogen activator inhibitor, and an increase in the number, activation, and aggregation of platelets.[12]
Chronic intranasal usage can degrade the cartilage separating the nostrils (the septum nasi), leading eventually to its complete disappearance. Due to the absorption of the cocaine from cocaine hydrochloride, the remaining hydrochloride forms a dilute hydrochloric acid.[84]
Illicitly-sold cocaine may be contaminated with levamisole.[85] Levamisole may accentuate cocaine's effects.[86] Levamisole-adulterated cocaine has been associated with autoimmune disease.[87]
Cocaine use leads to an increased risk of hemorrhagic and ischemic strokes.[48] Cocaine use also increases the risk of having a heart attack.[88]
Addiction
Relatives of persons with cocaine addiction have an increased risk of cocaine addiction.[89] Cocaine addiction occurs through ΔFosB overexpression in the nucleus accumbens, which results in altered transcriptional regulation in neurons within the nucleus accumbens. ΔFosB levels have been found to increase upon the use of cocaine.[90] Each subsequent dose of cocaine continues to increase ΔFosB levels with no ceiling of tolerance. Elevated levels of ΔFosB leads to increases in brain-derived neurotrophic factor (BDNF) levels, which in turn increases the number of dendritic branches and spines present on neurons involved with the nucleus accumbens and prefrontal cortex areas of the brain. This change can be identified rather quickly, and may be sustained weeks after the last dose of the drug.
Transgenic mice exhibiting inducible expression of ΔFosB primarily in the nucleus accumbens and dorsal striatum exhibit sensitized behavioural responses to cocaine.[91] They self-administer cocaine at lower doses than control,[92] but have a greater likelihood of relapse when the drug is withheld.[92][93] ΔFosB increases the expression of AMPA receptor subunit GluR2[91] and also decreases expression of dynorphin, thereby enhancing sensitivity to reward.[93]
DNA damage is increased in the brain of rodents by administration of cocaine.[94][95] During DNA repair of such damages, persistent chromatin alterations may occur such as methylation of DNA or the acetylation or methylation of histones at the sites of repair.[96] These alterations can be epigenetic scars in the chromatin that contribute to the persistent epigenetic changes found in cocaine addiction.
In humans, cocaine abuse may cause structural changes in brain connectivity, though it is unclear to what extent these changes are permanent.[97]
Dependence and withdrawal
Cocaine dependence develops after even brief periods of regular cocaine use[98] and produces a withdrawal state with emotional-motivational deficits upon cessation of cocaine use.
During pregnancy
Crack baby is a term for a child born to a mother who used crack cocaine during her pregnancy. The threat that cocaine use during pregnancy poses to the fetus is now considered exaggerated.[99] Studies show that prenatal cocaine exposure (independent of other effects such as, for example, alcohol, tobacco, or physical environment) has no appreciable effect on childhood growth and development.[100] In 2007, he National Institute on Drug Abuse of the United States warned about health risks while cautioning against stereotyping:
Many recall that "crack babies", or babies born to mothers who used crack cocaine while pregnant, were at one time written off by many as a lost generation. They were predicted to suffer from severe, irreversible damage, including reduced intelligence and social skills. It was later found that this was a gross exaggeration. However, the fact that most of these children appear normal should not be over-interpreted as indicating that there is no cause for concern. Using sophisticated technologies, scientists are now finding that exposure to cocaine during fetal development may lead to subtle, yet significant, later deficits in some children, including deficits in some aspects of cognitive performance, information-processing, and attention to tasks—abilities that are important for success in school.[101]
There are also warnings about the threat of breastfeeding: The March of Dimes said "it is likely that cocaine will reach the baby through breast milk," and advises the following regarding cocaine use during pregnancy:
Cocaine use during pregnancy can affect a pregnant woman and her unborn baby in many ways. During the early months of pregnancy, it may increase the risk of miscarriage. Later in pregnancy, it can trigger preterm labor (labor that occurs before 37 weeks of pregnancy) or cause the baby to grow poorly. As a result, cocaine-exposed babies are more likely than unexposed babies to be born with low birth weight (less than 5.5 lb or 2.5 kg). Low-birthweight babies are 20 times more likely to die in their first month of life than normal-weight babies, and face an increased risk of lifelong disabilities such as mental retardation and cerebral palsy. Cocaine-exposed babies also tend to have smaller heads, which generally reflect smaller brains. Some studies suggest that cocaine-exposed babies are at increased risk of birth defects, including urinary tract defects and, possibly, heart defects. Cocaine also may cause an unborn baby to have a stroke, irreversible brain damage, or a heart attack.[102]
Mortality
Persons with regular or problematic use of cocaine have a significantly higher rate of death, and are specifically at higher risk of traumatic deaths and deaths attributable to infectious disease.[103]
Pharmacology
Pharmacokinetics
The extent of absorption of cocaine into the systemic circulation after nasal insufflation is similar to that after oral ingestion. The rate of absorption after nasal insufflation is limited by cocaine-induced vasoconstriction of capillaries in the nasal mucosa. Onset of absorption after oral ingestion is delayed because cocaine is a weak base with a pKa of 8.6, and is thus in an ionized form that is poorly absorbed from the acidic stomach and easily absorbed from the alkaline duodenum.[11] The rate and extent of absorption from inhalation of cocaine is similar or greater than with intravenous injection, as inhalation provides access directly to the pulmonary capillary bed. The delay in absorption after oral ingestion may account for the popular belief that cocaine bioavailability from the stomach is lower than after insufflation. Compared with ingestion, the faster absorption of insufflated cocaine results in quicker attainment of maximum drug effects. Snorting cocaine produces maximum physiological effects within 40 minutes and maximum psychotropic effects within 20 minutes. Physiological and psychotropic effects from nasally insufflated cocaine are sustained for approximately 40–60 minutes after the peak effects are attained.[104]
Cocaine crosses the blood–brain barrier via both a proton-coupled organic cation antiporter[18][19] and (to a lesser extent) via passive diffusion across cell membranes.[20] As of September 2022, the gene or genes encoding the human proton-organic cation antiporter had not been identified.[105]
Cocaine has a short elimination half life of 0.7–1.5 hours and is extensively metabolized by plasma esterases and also by liver cholinesterases, with only about 1% excreted unchanged in the urine.[12] The metabolism is dominated by hydrolytic ester cleavage, so the eliminated metabolites consist mostly of benzoylecgonine (BE), the major metabolite, and other metabolites in lesser amounts such as ecgonine methyl ester (EME) and ecgonine.[106][12] Further minor metabolites of cocaine include norcocaine, p-hydroxycocaine, m-hydroxycocaine, p-hydroxybenzoylecgonine (pOHBE), and m-hydroxybenzoylecgonine.[107] If consumed with alcohol, cocaine combines with alcohol in the liver to form cocaethylene.[12] Studies have suggested cocaethylene is more euphoric, and has a higher cardiovascular toxicity than cocaine by itself.[12]
Depending on liver and kidney functions, cocaine metabolites are detectable in urine between three and eight days. Generally speaking benzoylecgonine is eliminated from someone's urine between three and five days. In urine from heavy cocaine users, benzoylecgonine can be detected within four hours after intake and in concentrations greater than 150 ng/mL for up to eight days later.[108]
Detection of cocaine metabolites in hair is possible in regular users until after the sections of hair grown during the period of cocaine use are cut or fall out.[109]
Pharmacodynamics
The pharmacodynamics of cocaine involve the complex relationships of neurotransmitters (inhibiting monoamine uptake in rats with ratios of about: serotonin:dopamine = 2:3, serotonin:norepinephrine = 2:5).[110][15] The most extensively studied effect of cocaine on the central nervous system is the blockade of the dopamine transporter protein. Dopamine neurotransmitter released during neural signaling is normally recycled via the transporter; i.e., the transporter binds the transmitter and pumps it out of the synaptic cleft back into the presynaptic neuron, where it is taken up into storage vesicles. Cocaine binds tightly at the dopamine transporter forming a complex that blocks the transporter's function. The dopamine transporter can no longer perform its reuptake function, and thus dopamine accumulates in the synaptic cleft. The increased concentration of dopamine in the synapse activates post-synaptic dopamine receptors, which makes the drug rewarding and promotes the compulsive use of cocaine.[111]
Cocaine affects certain serotonin (5-HT) receptors; in particular, it has been shown to antagonize the 5-HT3 receptor, which is a ligand-gated ion channel. An overabundance of 5-HT3 receptors is reported in cocaine-conditioned rats, though 5-HT3's role is unclear.[112] The 5-HT2 receptor (particularly the subtypes 5-HT2A, 5-HT2B and 5-HT2C) are involved in the locomotor-activating effects of cocaine.[113]
Cocaine has been demonstrated to bind as to directly stabilize the DAT transporter on the open outward-facing conformation. Further, cocaine binds in such a way as to inhibit a hydrogen bond innate to DAT. Cocaine's binding properties are such that it attaches so this hydrogen bond will not form and is blocked from formation due to the tightly locked orientation of the cocaine molecule. Research studies have suggested that the affinity for the transporter is not what is involved in the habituation of the substance so much as the conformation and binding properties to where and how on the transporter the molecule binds.[114]
Conflicting findings have challenged the widely accepted view that cocaine functions solely as a reuptake inhibitor. To induce euphoria an intravenous dose of 0.3-0.6 mg/kg of cocaine is required, which blocks 66-70% of dopamine transporters (DAT) in the brain.[115] Re-administering cocaine beyond this threshold does not significantly increase DAT occupancy but still results in an increase of euphoria which cannot be explained by reuptake inhibition alone. This discrepancy is not shared with other dopamine reuptake inhbitors like bupropion, sibutramine, mazindol or tesofensine, which have similar or higher potencies than cocaine as dopamine reuptake inhibitors. These findings have evoked a hypothesis that cocaine may also function as a so-called "DAT inverse agonist" or "negative allosteric modifier of DAT" resulting in dopamine transporter reversal, and subsequent dopamine release into the synaptic cleft from the axon terminal in a manner similar to but distinct from amphetamines.[116]
Sigma receptors are affected by cocaine, as cocaine functions as a sigma ligand agonist.[117] Further specific receptors it has been demonstrated to function on are NMDA and the D1 dopamine receptor.[118]
Cocaine also blocks sodium channels, thereby interfering with the propagation of action potentials;[119][75] thus, like lignocaine and novocaine, it acts as a local anesthetic. It also functions on the binding sites to the dopamine and serotonin sodium dependent transport area as targets as separate mechanisms from its reuptake of those transporters; unique to its local anesthetic value which makes it in a class of functionality different from both its own derived phenyltropanes analogues which have that removed. In addition to this, cocaine has some target binding to the site of the κ-opioid receptor.[120] Cocaine also causes vasoconstriction, thus reducing bleeding during minor surgical procedures. Recent research points to an important role of circadian mechanisms[121] and clock genes[122] in behavioral actions of cocaine.
Cocaine is known to suppress hunger and appetite by increasing co-localization of sigma σ1R receptors and ghrelin GHS-R1a receptors at the neuronal cell surface, thereby increasing ghrelin-mediated signaling of satiety[123] and possibly via other effects on appetitive hormones.[124] Chronic users may lose their appetite and can experience severe malnutrition and significant weight loss.
Cocaine effects, further, are shown to be potentiated for the user when used in conjunction with new surroundings and stimuli, and otherwise novel environs.[125]
Chemistry
Appearance


Cocaine in its purest form is a white, pearly product. Cocaine appearing in powder form is a salt, typically cocaine hydrochloride. Street cocaine is often adulterated or "cut" with talc, lactose, sucrose, glucose, mannitol, inositol, caffeine, procaine, phencyclidine, phenytoin, lignocaine, strychnine, levamisole, amphetamine, or heroin.[126][dubious – discuss]
Crack cocaine looks like irregular shaped white rocks.[127]
Forms
Salts
Cocaine — a tropane alkaloid — is a weakly alkaline compound, and can therefore combine with acidic compounds to form salts. The hydrochloride (HCl) salt of cocaine is by far the most commonly encountered, although the sulfate (SO42−) and the nitrate (NO3−) salts are occasionally seen. Different salts dissolve to a greater or lesser extent in various solvents — the hydrochloride salt is polar in character and is quite soluble in water.[128]
Base
As the name implies, "freebase" is the base form of cocaine, as opposed to the salt form. It is practically insoluble in water whereas hydrochloride salt is water-soluble.
Smoking freebase cocaine has the additional effect of releasing methylecgonidine into the user's system due to the pyrolysis of the substance (a side effect which insufflating or injecting powder cocaine does not create). Some research suggests that smoking freebase cocaine can be even more cardiotoxic than other routes of administration[129] because of methylecgonidine's effects on lung tissue[130] and liver tissue.[131]
Pure cocaine is prepared by neutralizing its compounding salt with an alkaline solution, which will precipitate non-polar basic cocaine. It is further refined through aqueous-solvent liquid–liquid extraction.
Crack cocaine

Crack is usually smoked in a glass pipe, and once inhaled, it passes from the lungs directly to the central nervous system, producing an almost immediate "high" that can be very powerful – this initial crescendo of stimulation is known as a "rush". This is followed by an equally intense low, leaving the user craving more of the drug. Addiction to crack usually occurs within four to six weeks - much more rapidly than regular cocaine.[132]
Powder cocaine (cocaine hydrochloride) must be heated to a high temperature (about 197 °C), and considerable decomposition/burning occurs at these high temperatures. This effectively destroys some of the cocaine and yields a sharp, acrid, and foul-tasting smoke. Cocaine base/crack can be smoked because it vaporizes with little or no decomposition at 98 °C (208 °F),[133] which is below the boiling point of water.
Crack is a lower purity form of free-base cocaine that is usually produced by neutralization of cocaine hydrochloride with a solution of baking soda (sodium bicarbonate, NaHCO3) and water, producing a very hard/brittle, off-white-to-brown colored, amorphous material that contains sodium carbonate, entrapped water, and other by-products as the main impurities. The origin of the name "crack" comes from the "crackling" sound (and hence the onomatopoeic moniker "crack") that is produced when the cocaine and its impurities (i.e. water, sodium bicarbonate) are heated past the point of vaporization.[134]
Coca leaf infusions
Coca herbal infusion (also referred to as coca tea) is used in coca-leaf producing countries much as any herbal medicinal infusion would elsewhere in the world. The free and legal commercialization of dried coca leaves under the form of filtration bags to be used as "coca tea" has been actively promoted by the governments of Peru and Bolivia for many years as a drink having medicinal powers. In Peru, the National Coca Company, a state-run corporation, sells cocaine-infused teas and other medicinal products and also exports leaves to the U.S. for medicinal use.[135]
Visitors to the city of Cuzco in Peru, and La Paz in Bolivia are greeted with the offering of coca leaf infusions (prepared in teapots with whole coca leaves) purportedly to help the newly arrived traveler overcome the malaise of high altitude sickness.[136] The effects of drinking coca tea are mild stimulation and mood lift.[137] It has also been promoted as an adjuvant for the treatment of cocaine dependence. One study on coca leaf infusion used with counseling in the treatment of 23 addicted coca-paste smokers in Lima, Peru found that the relapses rate fell from 4.35 times per month on average before coca tea treatment to one during treatment. The duration of abstinence increased from an average of 32 days before treatment to 217.2 days during treatment. This suggests that coca leaf infusion plus counseling may be effective at preventing relapse during cocaine addiction treatment.[138]
There is little information on the pharmacological and toxicological effects of consuming coca tea. A chemical analysis by solid-phase extraction and gas chromatography–mass spectrometry (SPE-GC/MS) of Peruvian and Bolivian tea bags indicated the presence of significant amounts of cocaine, the metabolite benzoylecgonine, ecgonine methyl ester and trans-cinnamoylcocaine in coca tea bags and coca tea. Urine specimens were also analyzed from an individual who consumed one cup of coca tea and it was determined that enough cocaine and cocaine-related metabolites were present to produce a positive drug test.[139]
Synthesis
Biosynthesis

The first synthesis and elucidation of the cocaine molecule was by Richard Willstätter in 1898.[140] Willstätter's synthesis derived cocaine from tropinone. Since then, Robert Robinson and Edward Leete have made significant contributions to the mechanism of the synthesis. (-NO3)
The additional carbon atoms required for the synthesis of cocaine are derived from acetyl-CoA, by addition of two acetyl-CoA units to the N-methyl-Δ1-pyrrolinium cation.[141] The first addition is a Mannich-like reaction with the enolate anion from acetyl-CoA acting as a nucleophile toward the pyrrolinium cation. The second addition occurs through a Claisen condensation. This produces a racemic mixture of the 2-substituted pyrrolidine, with the retention of the thioester from the Claisen condensation. In formation of tropinone from racemic ethyl [2,3-13C2]4(Nmethyl-2-pyrrolidinyl)-3-oxobutanoate there is no preference for either stereoisomer.[142]
In cocaine biosynthesis, only the (S)-enantiomer can cyclize to form the tropane ring system of cocaine. The stereoselectivity of this reaction was further investigated through study of prochiral methylene hydrogen discrimination.[143] This is due to the extra chiral center at C-2.[144] This process occurs through an oxidation, which regenerates the pyrrolinium cation and formation of an enolate anion, and an intramolecular Mannich reaction. The tropane ring system undergoes hydrolysis, SAM-dependent methylation, and reduction via NADPH for the formation of methylecgonine. The benzoyl moiety required for the formation of the cocaine diester is synthesized from phenylalanine via cinnamic acid.[145] Benzoyl-CoA then combines the two units to form cocaine.

N-methyl-pyrrolinium cation
The biosynthesis begins with L-Glutamine, which is derived to L-ornithine in plants. The major contribution of L-ornithine and L-arginine as a precursor to the tropane ring was confirmed by Edward Leete.[146] Ornithine then undergoes a pyridoxal phosphate-dependent decarboxylation to form putrescine. In some animals, the urea cycle derives putrescine from ornithine. L-ornithine is converted to L-arginine,[147] which is then decarboxylated via PLP to form agmatine. Hydrolysis of the imine derives N-carbamoylputrescine followed with hydrolysis of the urea to form putrescine. The separate pathways of converting ornithine to putrescine in plants and animals have converged. A SAM-dependent N-methylation of putrescine gives the N-methylputrescine product, which then undergoes oxidative deamination by the action of diamine oxidase to yield the aminoaldehyde. Schiff base formation confirms the biosynthesis of the N-methyl-Δ1-pyrrolinium cation.

Robert Robinson's acetonedicarboxylate
The biosynthesis of the tropane alkaloid is still not understood. Hemscheidt proposes that Robinson's acetonedicarboxylate emerges as a potential intermediate for this reaction.[148] Condensation of N-methylpyrrolinium and acetonedicarboxylate would generate the oxobutyrate. Decarboxylation leads to tropane alkaloid formation.

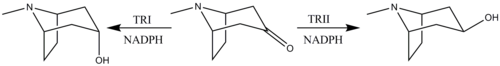
Reduction of tropinone
The reduction of tropinone is mediated by NADPH-dependent reductase enzymes, which have been characterized in multiple plant species.[149] These plant species all contain two types of the reductase enzymes, tropinone reductase I and tropinone reductase II. TRI produces tropine and TRII produces pseudotropine. Due to differing kinetic and pH/activity characteristics of the enzymes and by the 25-fold higher activity of TRI over TRII, the majority of the tropinone reduction is from TRI to form tropine.[150]
GMO synthesis
Research
In 2022, a GMO produced N. benthamiana were discovered that were able to produce 25% of the amount of cocaine found in a coca plant.[151]
Detection in body fluids
Cocaine and its major metabolites may be quantified in blood, plasma, or urine to monitor for use, confirm a diagnosis of poisoning, or assist in the forensic investigation of a traffic or other criminal violation or sudden death. Most commercial cocaine immunoassay screening tests cross-react appreciably with the major cocaine metabolites, but chromatographic techniques can easily distinguish and separately measure each of these substances. When interpreting the results of a test, it is important to consider the cocaine usage history of the individual, since a chronic user can develop tolerance to doses that would incapacitate a cocaine-naive individual, and the chronic user often has high baseline values of the metabolites in his system. Cautious interpretation of testing results may allow a distinction between passive or active usage, and between smoking versus other routes of administration.[152]
Field analysis
Cocaine may be detected by law enforcement using the Scott reagent. The test can easily generate false positives for common substances and must be confirmed with a laboratory test.[153][154]
Approximate cocaine purity can be determined using 1 mL 2% cupric sulfate pentahydrate in dilute HCl, 1 mL 2% potassium thiocyanate and 2 mL of chloroform. The shade of brown shown by the chloroform is proportional to the cocaine content. This test is not cross sensitive to heroin, methamphetamine, benzocaine, procaine and a number of other drugs but other chemicals could cause false positives.[155]
Usage
| Substance | Best estimate |
Low estimate |
High estimate |
|---|---|---|---|
| Amphetamine- type stimulants |
34.16 | 13.42 | 55.24 |
| Cannabis | 192.15 | 165.76 | 234.06 |
| Cocaine | 18.20 | 13.87 | 22.85 |
| Ecstasy | 20.57 | 8.99 | 32.34 |
| Opiates | 19.38 | 13.80 | 26.15 |
| Opioids | 34.26 | 27.01 | 44.54 |
According to a 2016 United Nations report, England and Wales are the countries with the highest rate of cocaine usage (2.4% of adults in the previous year).[157] Other countries where the usage rate meets or exceeds 1.5% are Spain and Scotland (2.2%), the United States (2.1%), Australia (2.1%), Uruguay (1.8%), Brazil (1.75%), Chile (1.73%), the Netherlands (1.5%) and Ireland (1.5%).[157]
Europe
Cocaine is the second most popular illegal recreational drug in Europe (behind cannabis). Since the mid-1990s, overall cocaine usage in Europe has been on the rise, but usage rates and attitudes tend to vary between countries. European countries with the highest usage rates are the United Kingdom, Spain, Italy, and the Republic of Ireland.
Approximately 17 million Europeans (5.1%) have used cocaine at least once and 3.5 million (1.1%) in the last year. About 1.9% (2.3 million) of young adults (15–34 years old) have used cocaine in the last year (latest data available as of 2018).[158]
Usage is particularly prevalent among this demographic: 4% to 7% of males have used cocaine in the last year in Spain, Denmark, the Republic of Ireland, Italy, and the United Kingdom. The ratio of male to female users is approximately 3.8:1, but this statistic varies from 1:1 to 13:1 depending on country.[159]
In 2014 London had the highest amount of cocaine in its sewage out of 50 European cities.[160]
United States
Cocaine is the second most popular illegal recreational drug in the United States (behind cannabis)[161] and the U.S. is the world's largest consumer of cocaine.[162] Its users span over different ages, races, and professions. In the 1970s and 1980s, the drug became particularly popular in the disco culture as cocaine usage was very common and popular in many discos such as Studio 54.
Dependence treatment
History
Discovery

Indigenous peoples of South America have chewed the leaves of Erythroxylon coca—a plant that contains vital nutrients as well as numerous alkaloids, including cocaine—for over a thousand years.[163] The coca leaf was, and still is, chewed almost universally by some indigenous communities. The remains of coca leaves have been found with ancient Peruvian mummies, and pottery from the time period depicts humans with bulged cheeks, indicating the presence of something on which they are chewing.[164] There is also evidence that these cultures used a mixture of coca leaves and saliva as an anesthetic for the performance of trepanation.[165]
When the Spanish arrived in South America, the conquistadors at first banned coca as an "evil agent of devil". But after discovering that without the coca the locals were barely able to work, the conquistadors legalized and taxed the leaf, taking 10% off the value of each crop.[166] In 1569, Spanish botanist Nicolás Monardes described the indigenous peoples' practice of chewing a mixture of tobacco and coca leaves to induce "great contentment":
When they wished to make themselves drunk and out of judgment they chewed a mixture of tobacco and coca leaves which make them go as they were out of their wittes.[167]
In 1609, Padre Blas Valera wrote:
Coca protects the body from many ailments, and our doctors use it in powdered form to reduce the swelling of wounds, to strengthen broken bones, to expel cold from the body or prevent it from entering, and to cure rotten wounds or sores that are full of maggots. And if it does so much for outward ailments, will not its singular virtue have even greater effect in the entrails of those who eat it?[168]
Isolation and naming
Although the stimulant and hunger-suppressant properties of coca had been known for many centuries, the isolation of the cocaine alkaloid was not achieved until 1855. Various European scientists had attempted to isolate cocaine, but none had been successful for two reasons: the knowledge of chemistry required was insufficient at the time, and contemporary conditions of sea-shipping from South America would often degrade the quality of the cocaine in the plant samples available to European chemists by the time they arrived.[169] However, by 1855, the German chemist Friedrich Gaedcke successfully isolated the cocaine alkaloid for the first time.[170] Gaedcke named the alkaloid "erythroxyline", and published a description in the journal Archiv der Pharmazie.[171]
In 1856, Friedrich Wöhler asked Dr. Carl Scherzer, a scientist aboard the Novara (an Austrian frigate sent by Emperor Franz Joseph to circle the globe), to bring him a large amount of coca leaves from South America. In 1859, the ship finished its travels and Wöhler received a trunk full of coca. Wöhler passed on the leaves to Albert Niemann, a PhD student at the University of Göttingen in Germany, who then developed an improved purification process.[172]
Niemann described every step he took to isolate cocaine in his dissertation titled Über eine neue organische Base in den Cocablättern (On a New Organic Base in the Coca Leaves), which was published in 1860 and earned him his Ph.D. He wrote of the alkaloid's "colourless transparent prisms" and said that "Its solutions have an alkaline reaction, a bitter taste, promote the flow of saliva and leave a peculiar numbness, followed by a sense of cold when applied to the tongue." Niemann named the alkaloid "cocaine" from "coca" (from Quechua "kúka") + suffix "ine".[172][173]
The first synthesis and elucidation of the structure of the cocaine molecule was by Richard Willstätter in 1898.[140] It was the first biomimetic synthesis of an organic structure recorded in academic chemical literature.[174][175] The synthesis started from tropinone, a related natural product and took five steps.
Because of the former use of cocaine as a local anesthetic, a suffix "-caine" was later extracted and used to form names of synthetic local anesthetics.
Medicalization



With the discovery of this new alkaloid, Western medicine was quick to exploit the possible uses of this plant.
In 1879, Vassili von Anrep, of the University of Würzburg, devised an experiment to demonstrate the analgesic properties of the newly discovered alkaloid. He prepared two separate jars, one containing a cocaine-salt solution, with the other containing merely saltwater. He then submerged a frog's legs into the two jars, one leg in the treatment and one in the control solution, and proceeded to stimulate the legs in several different ways. The leg that had been immersed in the cocaine solution reacted very differently from the leg that had been immersed in saltwater.[176]
Karl Koller (a close associate of Sigmund Freud, who would write about cocaine later) experimented with cocaine for ophthalmic usage. In an infamous experiment in 1884, he experimented upon himself by applying a cocaine solution to his own eye and then pricking it with pins. His findings were presented to the Heidelberg Ophthalmological Society. Also in 1884, Jellinek demonstrated the effects of cocaine as a respiratory system anesthetic. In 1885, William Halsted demonstrated nerve-block anesthesia,[177] and James Leonard Corning demonstrated peridural anesthesia.[178] 1898 saw Heinrich Quincke use cocaine for spinal anesthesia.
Popularization

In 1859, an Italian doctor, Paolo Mantegazza, returned from Peru, where he had witnessed first-hand the use of coca by the local indigenous peoples. He proceeded to experiment on himself and upon his return to Milan, he wrote a paper in which he described the effects. In this paper, he declared coca and cocaine (at the time they were assumed to be the same) as being useful medicinally, in the treatment of "a furred tongue in the morning, flatulence, and whitening of the teeth."
A chemist named Angelo Mariani who read Mantegazza's paper became immediately intrigued with coca and its economic potential. In 1863, Mariani started marketing a wine called Vin Mariani, which had been treated with coca leaves, to become coca wine. The ethanol in wine acted as a solvent and extracted the cocaine from the coca leaves, altering the drink's effect. It contained 6 mg cocaine per ounce of wine, but Vin Mariani which was to be exported contained 7.2 mg per ounce, to compete with the higher cocaine content of similar drinks in the United States. A "pinch of coca leaves" was included in John Styth Pemberton's original 1886 recipe for Coca-Cola, though the company began using decocainized leaves in 1906 when the Pure Food and Drug Act was passed.
In 1879 cocaine began to be used to treat morphine addiction. Cocaine was introduced into clinical use as a local anesthetic in Germany in 1884, about the same time as Sigmund Freud published his work Über Coca,[180] in which he wrote that cocaine causes:[181]
Exhilaration and lasting euphoria, which in no way differs from the normal euphoria of the healthy person. You perceive an increase of self-control and possess more vitality and capacity for work. In other words, you are simply normal, and it is soon hard to believe you are under the influence of any drug. Long intensive physical work is performed without any fatigue. This result is enjoyed without any of the unpleasant after-effects that follow exhilaration brought about by alcoholic beverages. No craving for the further use of cocaine appears after the first, or even after repeated taking of the drug.[182]
By 1885 the U.S. manufacturer Parke-Davis sold coca-leaf cigarettes and cheroots, a cocaine inhalant, a Coca Cordial, cocaine crystals, and cocaine solution for intravenous injection.[183] The company promised that its cocaine products would "supply the place of food, make the coward brave, the silent eloquent and render the sufferer insensitive to pain."

By the late Victorian era, cocaine use had appeared as a vice in literature. For example, it was injected by Arthur Conan Doyle's fictional Sherlock Holmes, generally to offset the boredom he felt when he was not working on a case.
In early 20th-century Memphis, Tennessee, cocaine was sold in neighborhood drugstores on Beale Street, costing five or ten cents for a small boxful. Stevedores along the Mississippi River used the drug as a stimulant, and white employers encouraged its use by black laborers.[184]
In 1909, Ernest Shackleton took "Forced March" brand cocaine tablets to Antarctica, as did Captain Scott a year later on his ill-fated journey to the South Pole.[185]
In the 1931 song "Minnie the Moocher", Cab Calloway heavily references cocaine use. He uses the phrase "kicking the gong around", slang for cocaine use; describes titular character Minnie as "tall and skinny;" and describes Smokey Joe as "cokey".[186] In the 1932 comedy musical film The Big Broadcast, Cab Calloway performs the song with his orchestra and mimes snorting cocaine in between verses.[187]
During the mid-1940s, amidst World War II, cocaine was considered for inclusion as an ingredient of a future generation of 'pep pills' for the German military, code named D-IX.[188]
In modern popular culture, references to cocaine are common. The drug has a glamorous image associated with the wealthy, famous and powerful, and is said to make users "feel rich and beautiful".[189][190][191][192] In addition the pace of modern society − such as in finance − gives many the incentive to make use of the drug.[189]

Modern usage

In many countries, cocaine is a popular recreational drug. Cocaine use is prevalent across all socioeconomic strata, including age, demographics, economic, social, political, religious, and livelihood.[193]
In the United States, the development of "crack" cocaine introduced the substance to a generally poorer inner-city market. The use of the powder form has stayed relatively constant, experiencing a new height of use across the 1980s and 1990s in the U.S.[194][195] However, from 2006 to 2010 cocaine use in the US declined by roughly half before again rising once again from 2017 onwards.[196] In the UK, cocaine use increased significantly between the 1990s and late 2000s, with a similar high consumption in some other European countries, including Spain.[197]
The estimated U.S. cocaine market exceeded US$70 billion in street value for the year 2005, exceeding revenues by corporations such as Starbucks.[198][199] Cocaine's status as a club drug shows its immense popularity among the "party crowd".[193]
In 1995 the World Health Organization (WHO) and the United Nations Interregional Crime and Justice Research Institute (UNICRI) announced in a press release the publication of the results of the largest global study on cocaine use ever undertaken. An American representative in the World Health Assembly banned the publication of the study, because it seemed to make a case for the positive uses of cocaine. An excerpt of the report strongly conflicted with accepted paradigms, for example, "that occasional cocaine use does not typically lead to severe or even minor physical or social problems." In the sixth meeting of the B committee, the US representative threatened that "If World Health Organization activities relating to drugs failed to reinforce proven drug control approaches, funds for the relevant programs should be curtailed". This led to the decision to discontinue publication. A part of the study was recuperated and published in 2010, including profiles of cocaine use in 20 countries, but are unavailable as of 2015[update].[200]
In October 2010 it was reported that the use of cocaine in Australia has doubled since monitoring began in 2003.[201]
A problem with illegal cocaine use, especially in the higher volumes used to combat fatigue (rather than increase euphoria) by long-term users, is the risk of ill effects or damage caused by the compounds used in adulteration. Cutting or "stepping on" the drug is commonplace, using compounds which simulate ingestion effects, such as Novocain (procaine) producing temporary anesthesia, as many users believe a strong numbing effect is the result of strong and/or pure cocaine, ephedrine or similar stimulants that are to produce an increased heart rate. The normal adulterants for profit are inactive sugars, usually mannitol, creatine, or glucose, so introducing active adulterants gives the illusion of purity and to 'stretch' or make it so a dealer can sell more product than without the adulterants, however the purity of the cocaine is subsequently lowered.[202][203] The adulterant of sugars allows the dealer to sell the product for a higher price because of the illusion of purity and allows the sale of more of the product at that higher price, enabling dealers to significantly increase revenue with little additional cost for the adulterants. A 2007 study by the European Monitoring Centre for Drugs and Drug Addiction showed that the purity levels for street purchased cocaine was often under 5% and on average under 50% pure.[204]
Society and culture
Legal status
The production, distribution, and sale of cocaine products is restricted (and illegal in most contexts) in most countries as regulated by the Single Convention on Narcotic Drugs, and the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances. In the United States the manufacture, importation, possession, and distribution of cocaine are additionally regulated by the 1970 Controlled Substances Act.
Some countries, such as Peru and Bolivia, permit the cultivation of coca leaf for traditional consumption by the local indigenous population, but nevertheless, prohibit the production, sale, and consumption of cocaine.[205] The provisions as to how much a coca farmer can yield annually is protected by laws such as the Bolivian Cato accord.[206] In addition, some parts of Europe, the United States, and Australia allow processed cocaine for medicinal uses only.
Australia
Cocaine is a Schedule 8 controlled drug in Australia under the Poisons Standard.[207] It is the second most popular illicit recreational drug in Australia behind cannabis.[208]
In Western Australia under the Misuse of Drugs Act 1981 4.0g of cocaine is the amount of prohibited drugs determining a court of trial, 2.0g is the amount of cocaine required for the presumption of intention to sell or supply and 28.0g is the amount of cocaine required for purposes of drug trafficking.[209]
United States

The US federal government instituted a national labeling requirement for cocaine and cocaine-containing products through the Pure Food and Drug Act of 1906.[210] The next important federal regulation was the Harrison Narcotics Tax Act of 1914. While this act is often seen as the start of prohibition, the act itself was not actually a prohibition on cocaine, but instead set up a regulatory and licensing regime.[211] The Harrison Act did not recognize addiction as a treatable condition and therefore the therapeutic use of cocaine, heroin, or morphine to such individuals was outlawed – leading a 1915 editorial in the journal American Medicine to remark that the addict "is denied the medical care he urgently needs, open, above-board sources from which he formerly obtained his drug supply are closed to him, and he is driven to the underworld where he can get his drug, but of course, surreptitiously and in violation of the law."[212] The Harrison Act left manufacturers of cocaine untouched so long as they met certain purity and labeling standards.[213] Despite that cocaine was typically illegal to sell and legal outlets were rarer, the quantities of legal cocaine produced declined very little.[213] Legal cocaine quantities did not decrease until the Jones–Miller Act of 1922 put serious restrictions on cocaine manufactures.[213]
Before the early 1900s, the primary problem caused by cocaine use was portrayed by newspapers to be addiction, not violence or crime, and the cocaine user was represented as an upper or middle class White person. In 1914, The New York Times published an article titled "Negro Cocaine 'Fiends' Are a New Southern Menace", portraying Black cocaine users as dangerous and able to withstand wounds that would normally be fatal.[214] The Anti-Drug Abuse Act of 1986 mandated the same prison sentences for distributing 500 grams of powdered cocaine and just 5 grams of crack cocaine.[215] In the National Survey on Drug Use and Health, white respondents reported a higher rate of powdered cocaine use, and Black respondents reported a higher rate of crack cocaine use.[216]
Interdiction


In 2004, according to the United Nations, 589 tonnes of cocaine were seized globally by law enforcement authorities. Colombia seized 188 t, the United States 166 t, Europe 79 t, Peru 14 t, Bolivia 9 t, and the rest of the world 133 t.[217]
Production
Colombia is as of 2019 the world's largest cocaine producer, with production more than tripling since 2013.[218][219] Three-quarters of the world's annual yield of cocaine has been produced in Colombia, both from cocaine base imported from Peru (primarily the Huallaga Valley) and Bolivia and from locally grown coca. There was a 28% increase in the amount of potentially harvestable coca plants which were grown in Colombia in 1998. This, combined with crop reductions in Bolivia and Peru, made Colombia the nation with the largest area of coca under cultivation after the mid-1990s. Coca grown for traditional purposes by indigenous communities, a use which is still present and is permitted by Colombian laws, only makes up a small fragment of total coca production, most of which is used for the illegal drug trade.[220]
An interview with a coca farmer published in 2003 described a mode of production by acid-base extraction that has changed little since 1905. Roughly 625 pounds (283 kg) of leaves were harvested per hectare, six times per year. The leaves were dried for half a day, then chopped into small pieces with a string trimmer and sprinkled with a small amount of powdered cement (replacing sodium carbonate from former times). Several hundred pounds of this mixture were soaked in 50 US gallons (190 L) of gasoline for a day, then the gasoline was removed and the leaves were pressed for the remaining liquid, after which they could be discarded. Then battery acid (weak sulfuric acid) was used, one bucket per 55 lb (25 kg) of leaves, to create a phase separation in which the cocaine free base in the gasoline was acidified and extracted into a few buckets of "murky-looking smelly liquid". Once powdered caustic soda was added to this, the cocaine precipitated and could be removed by filtration through a cloth. The resulting material, when dried, was termed pasta and sold by the farmer. The 3,750 pounds (1,700 kg) yearly harvest of leaves from a hectare produced 6 lb (2.5 kg) of pasta, approximately 40–60% cocaine. Repeated recrystallization from solvents, producing pasta lavada and eventually crystalline cocaine were performed at specialized laboratories after the sale.[221]
Attempts to eradicate coca fields through the use of defoliants have devastated part of the farming economy in some coca-growing regions of Colombia, and strains appear to have been developed that are more resistant or immune to their use. Whether these strains are natural mutations or the product of human tampering is unclear. These strains have also shown to be more potent than those previously grown, increasing profits for the drug cartels responsible for the exporting of cocaine. Although production fell temporarily, coca crops rebounded in numerous smaller fields in Colombia, rather than the larger plantations.[222][223]
The cultivation of coca has become an attractive economic decision for many growers due to the combination of several factors, including the lack of other employment alternatives, the lower profitability of alternative crops in official crop substitution programs, the eradication-related damages to non-drug farms, the spread of new strains of the coca plant due to persistent worldwide demand.[224][225]
| 2000 | 2001 | 2002 | 2003 | 2004 | |
|---|---|---|---|---|---|
| Net cultivation km2 (sq mi) | 1,875 (724) | 2,218 (856) | 2,007.5 (775.1) | 1,663 (642) | 1,662 (642) |
| Potential pure cocaine production (tonnes) | 770 | 925 | 830 | 680 | 645 |
The latest estimate provided by the U.S. authorities on the annual production of cocaine in Colombia refers to 290 metric tons. As of the end of 2011, the seizure operations of Colombian cocaine carried out in different countries have totaled 351.8 metric tons of cocaine, i.e. 121.3% of Colombia's annual production according to the U.S. Department of State's estimates.[227][228]
Synthesis
Synthesizing cocaine could eliminate the high visibility and low reliability of offshore sources and international smuggling, replacing them with clandestine domestic laboratories, as are common for illicit methamphetamine, but is rarely done. Natural cocaine remains the lowest cost and highest quality supply of cocaine. Formation of inactive stereoisomers (cocaine has four chiral centres – 1R 2R, 3S, and 5S, two of them dependent, hence eight possible stereoisomers) plus synthetic by-products limits the yield and purity.[229][230]
Trafficking and distribution

Organized criminal gangs operating on a large scale dominate the cocaine trade. Most cocaine is grown and processed in South America, particularly in Colombia, Bolivia, Peru, and smuggled into the United States and Europe, the United States being the world's largest consumer of cocaine,[162] where it is sold at huge markups; usually in the US at $80–120 for 1 gram, and $250–300 for 3.5 grams (1/8 of an ounce, or an "eight ball").[231]
Caribbean and Mexican routes
The primary cocaine importation points in the United States have been in Arizona, southern California, southern Florida, and Texas. Typically, land vehicles are driven across the U.S.–Mexico border. Sixty-five percent of cocaine enters the United States through Mexico, and the vast majority of the rest enters through Florida.[232][page needed] As of 2015[update], the Sinaloa Cartel is the most active drug cartel involved in smuggling illicit drugs like cocaine into the United States and trafficking them throughout the United States.[233]
Cocaine traffickers from Colombia and Mexico have established a labyrinth of smuggling routes throughout the Caribbean, the Bahama Island chain, and South Florida. They often hire traffickers from Mexico or the Dominican Republic to transport the drug using a variety of smuggling techniques to U.S. markets. These include airdrops of 500 to 700 kg (1,100 to 1,500 lb) in the Bahama Islands or off the coast of Puerto Rico, mid-ocean boat-to-boat transfers of 500 to 2,000 kg (1,100 to 4,400 lb), and the commercial shipment of tonnes of cocaine through the port of Miami.[234][235][236]
Chilean route
Another route of cocaine traffic goes through Chile, which is primarily used for cocaine produced in Bolivia since the nearest seaports lie in northern Chile. The arid Bolivia–Chile border is easily crossed by 4×4 vehicles that then head to the seaports of Iquique and Antofagasta. While the price of cocaine is higher in Chile than in Peru and Bolivia, the final destination is usually Europe, especially Spain where drug dealing networks exist among South American immigrants."The Cocaine Pipeline to Europe" (PDF). Global Initiative. February 2021. Retrieved 29 June 2024.[237]
Techniques
Cocaine is also carried in small, concealed, kilogram quantities across the border by couriers known as "mules" (or "mulas"), who cross a border either legally, for example, through a port or airport, or illegally elsewhere. The drugs may be strapped to the waist or legs or hidden in bags, or hidden in the body (by swallowing or placement inside an orifice), typically known as 'bodypacking. If the mule gets through without being caught, the gangs will receive most of the profits. If the mule caught, gangs may sever all links and the mule will usually stand trial for trafficking alone.[238] In many cases, mules are often forced into the role, as result of coercion, violence, threats or extreme poverty.[238][239]
Bulk cargo ships are also used to smuggle cocaine to staging sites in the western Caribbean–Gulf of Mexico area. These vessels are typically 150–250-foot (50–80 m) coastal freighters that carry an average cocaine load of approximately 2.5 tonnes. Commercial fishing vessels are also used for smuggling operations. In areas with a high volume of recreational traffic, smugglers use the same types of vessels, such as go-fast boats, like those used by the local populations.[240][241]
Sophisticated drug subs are the latest tool drug runners are using to bring cocaine north from Colombia, it was reported on 20 March 2008. Although the vessels were once viewed as a quirky sideshow in the drug war, they are becoming faster, more seaworthy, and capable of carrying bigger loads of drugs than earlier models, according to those charged with catching them.[242]
Sales to consumers

Cocaine is readily available in all major countries' metropolitan areas. According to the Summer 1998 Pulse Check, published by the U.S. Office of National Drug Control Policy, cocaine use had stabilized across the country, with a few increases reported in San Diego, Bridgeport, Miami, and Boston. In the West, cocaine usage was lower, which was thought to be due to a switch to methamphetamine among some users; methamphetamine is cheaper, three and a half times more powerful, and lasts 12–24 times longer with each dose.[243][244] Nevertheless, the number of cocaine users remain high, with a large concentration among urban youth.
In addition to the amounts previously mentioned, cocaine can be sold in "bill sizes": As of 2007[update] for example, $10 might purchase a "dime bag", a very small amount (0.1–0.15 g) of cocaine. These amounts and prices are very popular among young people because they are inexpensive and easily concealed on one's body. Quality and price can vary dramatically depending on supply and demand, and on geographic region.[245]
In 2008, the European Monitoring Centre for Drugs and Drug Addiction reports that the typical retail price of cocaine varied between €50 and €75 per gram in most European countries, although Cyprus, Romania, Sweden, and Turkey reported much higher values.[246]
Consumption
World annual cocaine consumption, as of 2000, stood at around 600 tonnes, with the United States consuming around 300 t, 50% of the total, Europe about 150 t, 25% of the total, and the rest of the world the remaining 150 t or 25%.[247] It is estimated that 1.5 million people in the United States used cocaine in 2010, down from 2.4 million in 2006.[12] Conversely, cocaine use appears to be increasing[when?] in Europe with the highest prevalences in Spain, the United Kingdom, Italy, and Ireland.[12]
The 2010 UN World Drug Report concluded that "it appears that the North American cocaine market has declined in value from US$47 billion in 1998 to US$38 billion in 2008. Between 2006 and 2008, the value of the market remained basically stable".[248]
See also
- Black cocaine
- Coca alkaloids
- Coca eradication
- Cocaine and amphetamine regulated transcript
- Cocaine Anonymous
- Cocaine Cowboys
- Cocaine paste
- Crack cocaine § Crack lung
- Crack epidemic
- Illegal drug trade in Latin America
- Coca production in Colombia
- Clan del Golfo
- Legal status of cocaine
- List of cocaine analogues
- List of countries by prevalence of cocaine use
- Methylphenidate
- Modafinil
- Pre-Columbian trans-oceanic evidence for cocaine in ancient Egypt
- Prenatal cocaine exposure
- Route 36, cocaine bar in Bolivia
- Tranquilandia
- Ypadu
References
- ^ Nordegren T (2002). The A-Z Encyclopedia of Alcohol and Drug Abuse. Universal-Publishers. p. 461. ISBN 978-1-58112-404-0. Archived from the original on 8 July 2024. Retrieved 3 September 2020.
- ^ a b c "Goprelto – cocaine hydrochloride solution". DailyMed. 3 January 2020. Archived from the original on 30 July 2020. Retrieved 30 April 2020.
- ^ a b c "Numbrino – cocaine hydrochloride nasal solution". DailyMed. 28 February 2020. Archived from the original on 30 July 2020. Retrieved 30 April 2020.
- ^ Ghodse H (2010). Ghodse's Drugs and Addictive Behaviour: A Guide to Treatment (4 ed.). Cambridge University Press. p. 91. ISBN 978-1-139-48567-8. Archived from the original on 10 September 2017.
- ^ Introduction to Pharmacology (3 ed.). Abingdon: CRC Press. 2007. pp. 222–223. ISBN 978-1-4200-4742-4. Archived from the original on 10 September 2017.
- ^ a b Azizi SA (December 2020). "Monoamines: Dopamine, Norepinephrine, and Serotonin, Beyond Modulation, "Switches" That Alter the State of Target Networks". The Neuroscientist. 28 (2): 121–143. doi:10.1177/1073858420974336. ISSN 1073-8584. PMID 33292070. S2CID 228080727.
- ^ "DEA / Drug Scheduling". www.dea.gov. Archived from the original on 9 August 2017. Retrieved 7 August 2017.
- ^ a b Fattinger K, Benowitz NL, Jones RT, Verotta D (July 2000). "Nasal mucosal versus gastrointestinal absorption of nasally administered cocaine". European Journal of Clinical Pharmacology. 56 (4): 305–10. doi:10.1007/s002280000147. PMID 10954344. S2CID 20708443.
- ^ Barnett G, Hawks R, Resnick R (1981). "Cocaine pharmacokinetics in humans". Journal of Ethnopharmacology. 3 (2–3): 353–66. doi:10.1016/0378-8741(81)90063-5. PMID 7242115.
- ^ Jeffcoat AR, Perez-Reyes M, Hill JM, Sadler BM, Cook CE (1989). "Cocaine disposition in humans after intravenous injection, nasal insufflation (snorting), or smoking". Drug Metabolism and Disposition. 17 (2): 153–9. PMID 2565204.
- ^ a b Wilkinson P, Van Dyke C, Jatlow P, Barash P, Byck R (March 1980). "Intranasal and oral cocaine kinetics". Clinical Pharmacology and Therapeutics. 27 (3): 386–94. doi:10.1038/clpt.1980.52. PMID 7357795. S2CID 29851205.
- ^ a b c d e f g h i j k l m n o p q r s t Zimmerman JL (October 2012). "Cocaine intoxication". Critical Care Clinics. 28 (4): 517–26. doi:10.1016/j.ccc.2012.07.003. PMID 22998988.
- ^ "Cocaine". American Heritage Dictionary. Archived from the original on 3 January 2023. Retrieved 3 January 2023.
- ^ Plowman T (June 1982). "The identification of coca (Erythroxylum species): 1860–1910". Botanical Journal of the Linnean Society. 84 (4): 329–353. doi:10.1111/j.1095-8339.1982.tb00368.x.
- ^ a b c d e f Pomara C, Cassano T, D'Errico S, Bello S, Romano AD, Riezzo I, et al. (2012). "Data available on the extent of cocaine use and dependence: biochemistry, pharmacologic effects and global burden of disease of cocaine abusers". Current Medicinal Chemistry. 19 (33): 5647–57. doi:10.2174/092986712803988811. PMID 22856655.
- ^ Connors NJ, Hoffman RS (November 2013). "Experimental treatments for cocaine toxicity: a difficult transition to the bedside". The Journal of Pharmacology and Experimental Therapeutics. 347 (2): 251–7. doi:10.1124/jpet.113.206383. PMID 23978563. S2CID 6767268.
- ^ Hamdan AL, Sataloff RT, Hawkshaw MJ (2022). "Topical Anesthesia in Office-Based Laryngeal Surgery". Office-Based Laryngeal Surgery. USA: Springer. pp. 123–137. doi:10.1007/978-3-030-91936-8_6. ISBN 978-3-030-91935-1. Archived from the original on 18 July 2022. Retrieved 18 July 2022.
- ^ a b Sachkova A, Doetsch DA, Jensen O, Brockmöller J, Ansari S (October 2021). "How do psychostimulants enter the human brain? Analysis of the role of the proton-organic cation antiporter". Biochemical Pharmacology. 192: 114751. doi:10.1016/j.bcp.2021.114751. PMID 34464621.
- ^ a b Tega Y, Tabata H, Kurosawa T, Kitamura A, Itagaki F, Oshitari T, et al. (January 2021). "Structural Requirements for Uptake of Diphenhydramine Analogs into hCMEC/D3 Cells Via the Proton-Coupled Organic Cation Antiporter". Journal of Pharmaceutical Sciences. 110 (1): 397–403. doi:10.1016/j.xphs.2020.09.001. PMID 32898521.
- ^ a b Chapy H, Smirnova M, André P, Schlatter J, Chiadmi F, Couraud PO, et al. (October 2014). "Carrier-mediated cocaine transport at the blood–brain barrier as a putative mechanism in addiction liability". The International Journal of Neuropsychopharmacology. 18 (1): pyu001. doi:10.1093/ijnp/pyu001. PMC 4368859. PMID 25539501.
- ^ Cheng MH, Block E, Hu F, Cobanoglu MC, Sorkin A, Bahar I (2015). "Insights into the Modulation of Dopamine Transporter Function by Amphetamine, Orphenadrine, and Cocaine Binding". Frontiers in Neurology. 6: 134. doi:10.3389/fneur.2015.00134. PMC 4460958. PMID 26106364.
- ^ Proebstl L, Kamp F, Manz K, Krause D, Adorjan K, Pogarell O, et al. (June 2019). "Effects of stimulant drug use on the dopaminergic system: A systematic review and meta-analysis of in vivo neuroimaging studies". European Psychiatry. 59: 15–24. doi:10.1016/j.eurpsy.2019.03.003. PMID 30981746.
- ^ "How does cocaine produce its effects?". Archived from the original on 18 January 2022. Retrieved 12 May 2021.
- ^ Wise RA, Robble MA (January 2020). "Dopamine and Addiction". Annual Review of Psychology. 71 (1): 79–106. doi:10.1146/annurev-psych-010418-103337. PMID 31905114.
- ^ a b Ambre JJ, Belknap SM, Nelson J, Ruo TI, Shin SG, Atkinson AJ (July 1988). "Acute tolerance to cocaine in humans". Clinical Pharmacology and Therapeutics. 44 (1): 1–8. doi:10.1038/clpt.1988.104. PMID 3390996. S2CID 44253676.
- ^ Paludetto LS, Florence LL, Torales J, Ventriglio A, Castaldelli-Maia JM (29 March 2024). "Mapping the Neural Substrates of Cocaine Craving: A Systematic Review". Brain Sciences. 14 (4): 329. doi:10.3390/brainsci14040329. PMC 11048489. PMID 38671981.
- ^ Wolf ME (June 2016). "Synaptic mechanisms underlying persistent cocaine craving". Nature Reviews. Neuroscience. 17 (6): 351–365. doi:10.1038/nrn.2016.39. PMC 5466704. PMID 27150400.
- ^ Walsh SL, Stoops WW, Moody DE, Lin SN, Bigelow GE (August 2009). "Repeated dosing with oral cocaine in humans: assessment of direct effects, withdrawal, and pharmacokinetics". Experimental and Clinical Psychopharmacology. 17 (4): 205–216. doi:10.1037/a0016469. PMC 2811070. PMID 19653786.
- ^ Sordo L, Indave BI, Barrio G, Degenhardt L, de la Fuente L, Bravo MJ (September 2014). "Cocaine use and risk of stroke: a systematic review". Drug and Alcohol Dependence. 142: 1–13. doi:10.1016/j.drugalcdep.2014.06.041. PMID 25066468.
- ^ a b Goldstein RA, DesLauriers C, Burda AM (January 2009). "Cocaine: history, social implications, and toxicity--a review". Disease-a-Month. 55 (1): 6–38. doi:10.1016/j.disamonth.2008.10.002. PMID 19081448.
- ^ "Fentanyl-Adulterated Cocaine: Strategies To Address The New Normal". 25 April 2019. Archived from the original on 17 December 2022. Retrieved 17 December 2022.
- ^ Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. (GBD 2017 Causes of Death Collaborators) (November 2018). "Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017". Lancet. 392 (10159): 1736–1788. doi:10.1016/S0140-6736(18)32203-7. PMC 6227606. PMID 30496103.
- ^ Valdez LM, Taboada J, Valdez JE (June 2015). "Ancient Use of Coca Leaves in the Peruvian Central Highlands". Journal of Anthropological Research. 71 (2): 231–258. doi:10.3998/jar.0521004.0071.204. hdl:2027/spo.0521004.0071.204. S2CID 163842955.
- ^ Martin RT (October 1970). "The role of coca in the history, religion, and medicine of South American Indians". Economic Botany. 24 (4): 422–438. Bibcode:1970EcBot..24..422M. doi:10.1007/BF02860746. S2CID 34523519.
- ^ Plant T, Aref-Adib G (June 2008). "Travelling to new heights: practical high altitude medicine". British Journal of Hospital Medicine. 69 (6): 348–352. doi:10.12968/hmed.2008.69.6.29626. PMID 18646420.
- ^ World Drug Report 2021: Booklet 4 (PDF). [S.l.]: United Nations Office on Drugs and Crime. 2021. p. 35. ISBN 978-92-1-148361-1. Archived (PDF) from the original on 24 June 2021.
- ^ Room R, Reuter P (January 2012). "How well do international drug conventions protect public health?". Lancet. 379 (9810): 84–91. doi:10.1016/s0140-6736(11)61423-2. PMID 22225673. S2CID 23386203.
The international treaties have also constrained national policy experimentation because they require nation states to criminalise drug use
- ^ "Drug Fact Sheet: Cocaine" (PDF). Drug Enforcement Agency. Archived (PDF) from the original on 21 June 2020. Retrieved 17 June 2022.
- ^ "Drug Fact Sheet: Cocaine" (PDF). US Department for Justice and Drug Enforcement Administration. Archived (PDF) from the original on 21 June 2020. Retrieved 29 June 2024.
- ^ Berkowitz AL (2022). "Chapter 10: Pupillary Control & Approach to Anisocoria: Cranial Nerves 2 & 3". Clinical Neurology & Neuroanatomy: A Localization-Based Approach (Digital) (2nd ed.). McGraw Hill. ISBN 978-1260453362.
- ^ Dwyer C, Sowerby L, Rotenberg BW (August 2016). "Is cocaine a safe topical agent for use during endoscopic sinus surgery?". The Laryngoscope (Review). 126 (8): 1721–1723. doi:10.1002/lary.25836. PMID 27075241.
- ^ Latorre F, Klimek L (January 1999). "Does cocaine still have a role in nasal surgery?". Drug Safety. 20 (1): 9–13. doi:10.2165/00002018-199920010-00002. PMID 9935273. S2CID 40598106.
- ^ Benjamin E, Wong DK, Choa D (December 2004). "'Moffett's' solution: a review of the evidence and scientific basis for the topical preparation of the nose". Clinical Otolaryngology and Allied Sciences. 29 (6): 582–587. doi:10.1111/j.1365-2273.2004.00894.x. PMID 15533141.
- ^ "Drug Approval Package: Goprelto (cocaine hydrochloride)". U.S. Food and Drug Administration (FDA). 30 April 2018. Archived from the original on 28 July 2020. Retrieved 30 April 2020.
- ^
 This article incorporates text from this source, which is in the public domain: "Numbrino: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 28 July 2020. Retrieved 30 April 2020.
This article incorporates text from this source, which is in the public domain: "Numbrino: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 28 July 2020. Retrieved 30 April 2020.
- ^ a b World Health Organization (2004). Neuroscience of psychoactive substance use and dependence. World Health Organization. p. 89. ISBN 978-9241562355. Archived from the original on 30 April 2016.
- ^ World Health Organization (2007). International medical guide for ships. World Health Organization. p. 242. ISBN 978-9241547208. Archived from the original on 30 April 2016.
- ^ a b Sordo L, Indave BI, Barrio G, Degenhardt L, de la Fuente L, Bravo MJ (September 2014). "Cocaine use and risk of stroke: a systematic review". Drug and Alcohol Dependence (Systematic Review). 142: 1–13. doi:10.1016/j.drugalcdep.2014.06.041. PMID 25066468.
- ^ Donroe JH, Tetrault JM (July 2017). "Substance Use, Intoxication, and Withdrawal in the Critical Care Setting". Critical Care Clinics (Review). 33 (3): 543–558. doi:10.1016/j.ccc.2017.03.003. PMID 28601134.
- ^ Fehrman E, Egan V, Gorban AN, Levesley J, Mirkes EM, Muhammad AK (2019). Personality Traits and Drug Consumption. A Story Told by Data. Springer, Cham. arXiv:2001.06520. doi:10.1007/978-3-030-10442-9. ISBN 978-3-030-10441-2. S2CID 151160405.
- ^ "The tradition of chewing coca". Archived from the original on 6 May 2021. Retrieved 6 May 2021.
- ^ a b Luks AM, McIntosh SE, Grissom CK, Auerbach PS, Rodway GW, Schoene RB, et al. (June 2010). "Wilderness Medical Society consensus guidelines for the prevention and treatment of acute altitude illness" (PDF). Wilderness & Environmental Medicine. 21 (2): 146–155. doi:10.1016/j.wem.2010.03.002. PMID 20591379. S2CID 30571498. Archived from the original (PDF) on 15 October 2012. (mirror: [1])
- ^ Siegel RK, Elsohly MA, Plowman T, Rury PM, Jones RT (January 1986). "Cocaine in herbal tea". JAMA. 255 (1): 40. doi:10.1001/jama.1986.03370010042021. PMID 3940302.
- ^ "DrugFacts: Cocaine". National Institute on Drug Abuse. April 2013. Archived from the original on 11 July 2015. Retrieved 11 July 2015.
- ^ "The Dangers Of Snorting Cocaine (Insufflation)". Vertava Health. Archived from the original on 8 April 2022. Retrieved 25 February 2022.
- ^ a b c Volkow ND, Wang GJ, Fischman MW, Foltin R, Fowler JS, Franceschi D, et al. (August 2000). "Effects of route of administration on cocaine-induced dopamine transporter blockade in the human brain". Life Sciences. 67 (12): 1507–1515. doi:10.1016/S0024-3205(00)00731-1. PMID 10983846.
- ^ "Sniffing Around the History of the McDonald's 'Cocaine Spoon'". www.mentalfloss.com. 19 March 2021. Archived from the original on 8 July 2024. Retrieved 14 June 2021.
- ^ "Cocaine terminology". Archived from the original on 9 July 2007.
- ^ Bonkovsky HL, Mehta S (February 2001). "Hepatitis C: a review and update". Journal of the American Academy of Dermatology. 44 (2): 159–182. doi:10.1067/mjd.2001.109311. PMID 11174373.
- ^ Dimitrijevic N, Dzitoyeva S, Manev H (August 2004). "An automated assay of the behavioral effects of cocaine injections in adult Drosophila". Journal of Neuroscience Methods. 137 (2): 181–184. doi:10.1016/j.jneumeth.2004.02.023. PMID 15262059. S2CID 19882594.
- ^ "Appendix B: Production of Cocaine Hydrochloride and Cocaine Base". US Justice Dep. Archived from the original on 30 November 2009.
- ^ Garcia RC, Torres LH, Balestrin NT, Andrioli TC, Flório JC, de Oliveira CD, et al. (February 2017). "Anhydroecgonine methyl ester, a cocaine pyrolysis product, may contribute to cocaine behavioral sensitization". Toxicology. 376: 44–50. Bibcode:2017Toxgy.376...44G. doi:10.1016/j.tox.2016.04.009. PMID 27129946.
- ^ Reist M (16 January 2005). "A rose by another name: crack pipe". Lincoln Journal Star. Archived from the original on 26 July 2010. Retrieved 21 August 2009.
- ^ "Cocaine". www.talktofrank.com. Archived from the original on 2 February 2017. Retrieved 23 January 2017.
- ^ Nutt DJ, King LA, Phillips LD (November 2010). "Drug harms in the UK: a multicriteria decision analysis". Lancet. 376 (9752): 1558–65. CiteSeerX 10.1.1.690.1283. doi:10.1016/S0140-6736(10)61462-6. PMID 21036393. S2CID 5667719.
- ^ a b "Overdose Death Rates". By National Institute on Drug Abuse (NIDA). Archived from the original on 28 November 2015.
- ^ Nutt D, King LA, Saulsbury W, Blakemore C (March 2007). "Development of a rational scale to assess the harm of drugs of potential misuse". Lancet. 369 (9566): 1047–53. doi:10.1016/S0140-6736(07)60464-4. PMID 17382831. S2CID 5903121.
- ^ Heesch CM, Negus BH, Keffer JH, Snyder RW, Risser RC, Eichhorn EJ (August 1995). "Effects of cocaine on cortisol secretion in humans". The American Journal of the Medical Sciences. 310 (2): 61–4. doi:10.1097/00000441-199508000-00004. PMID 7631644. S2CID 11042810.
- ^ Pudiak CM, KuoLee R, Bozarth MA (July 2014). "Tolerance to cocaine in brain stimulation reward following continuous cocaine infusions". Pharmacology, Biochemistry, and Behavior. 122: 246–52. doi:10.1016/j.pbb.2014.04.006. PMID 24768900. S2CID 207332822.
- ^ Gullapalli BT, Natarajan A, Angarita GA, Malison RT, Ganesan D, Rahman T (21 June 2019). "On-body Sensing of Cocaine Craving, Euphoria and Drug-Seeking Behavior Using Cardiac and Respiratory Signals". Proceedings of the ACM on Interactive, Mobile, Wearable and Ubiquitous Technologies. 3 (2): 1–31. doi:10.1145/3328917. S2CID 195357215.
- ^ Calipari ES, Ferris MJ, Jones SR (January 2014). "Extended access of cocaine self-administration results in tolerance to the dopamine-elevating and locomotor-stimulating effects of cocaine". Journal of Neurochemistry. 128 (2): 224–32. doi:10.1111/jnc.12452. PMC 3947316. PMID 24102293.
- ^ a b Zhao W (2008). Mechanisms Mediating Sex Differences in the Effects of Cocaine. University of Michigan. p. 3. ISBN 978-0-549-99458-9. Archived from the original on 4 April 2014. Retrieved 25 September 2012.
- ^ Boles SM, Miotto K (March–April 2003). "Substance abuse and violence: A review of the literature". Aggression and Violent Behavior. 8 (2): 155–174. doi:10.1016/S1359-1789(01)00057-X.
- ^ Pergolizzi JV, Magnusson P, LeQuang JA, Breve F, Varrassi G (April 2021). "Cocaine and Cardiotoxicity: A Literature Review". Cureus. 13 (4): e14594. doi:10.7759/cureus.14594. ISSN 2168-8184. PMC 8136464. PMID 34036012.
- ^ Gohil H, Miskovic M, Buxton JA, Holland SP, Strike C (August 2021). "Smoke Gets in the Eye: A systematic review of case reports of ocular complications of crack cocaine use". Drug and Alcohol Review. 41 (2): 347–355. doi:10.1111/dar.13366. PMID 34337815. S2CID 236775586.
- ^ Frazer KM, Richards Q, Keith DR (August 2018). "The long-term effects of cocaine use on cognitive functioning: A systematic critical review". Behavioural Brain Research. 348: 241–262. doi:10.1016/j.bbr.2018.04.005. PMID 29673580. S2CID 4992738.
- ^ D'haenen H, den Boer JA, Willner P, eds. (2002). Biological Psychiatry. Vol. 2 (2 ed.). Wiley. p. 528. ISBN 978-0-471-49198-9.
- ^ Wang GJ, Volkow ND, Fowler JS, Fischman M, Foltin R, Abumrad NN, et al. (8 August 1997). "Cocaine abusers do not show loss of dopamine transporters with age". Life Sciences. 61 (11): 1059–65. doi:10.1016/s0024-3205(97)00614-0. PMID 9307051.
- ^ Little KY, Ramssen E, Welchko R, Volberg V, Roland CJ, Cassin B (August 2009). "Decreased brain dopamine cell numbers in human cocaine users". Psychiatry Research. 168 (3): 173–80. doi:10.1016/j.psychres.2008.10.034. PMID 19233481. S2CID 27618292.
- ^ Sharma HS, Muresanu D, Sharma A, Patnaik R (2009). "Cocaine-induced breakdown of the blood–brain barrier and neurotoxicity". International Review of Neurobiology. 88: 297–334. doi:10.1016/S0074-7742(09)88011-2. ISBN 978-0-12-374504-0. PMID 19897082.
- ^ Karch SB (2009). Karch's pathology of drug abuse (4 ed.). Boca Raton: CRC Press. p. 70. ISBN 978-0-8493-7881-2. Archived from the original on 10 September 2017.
- ^ Baigent M (2003). "Physical complications of substance abuse: what the psychiatrist needs to know". Curr Opin Psychiatry. 16 (3): 291–296. doi:10.1097/00001504-200305000-00004.
- ^ Pagliaro L, Pagliaro AM (2004). Pagliaros' Comprehensive Guide to Drugs and Substances of Abuse. Washington, D.C.: American Pharmacists Association. ISBN 978-1-58212-066-9.
- ^ Chang A, Osterloh J, Thomas J (September 2010). "Levamisole: a dangerous new cocaine adulterant". Clinical Pharmacology and Therapeutics. 88 (3): 408–11. doi:10.1038/clpt.2010.156. PMID 20668440. S2CID 31414939.
- ^ Tallarida CS, Egan E, Alejo GD, Raffa R, Tallarida RJ, Rawls SM (April 2014). "Levamisole and cocaine synergism: a prevalent adulterant enhances cocaine's action in vivo". Neuropharmacology. 79: 590–5. doi:10.1016/j.neuropharm.2014.01.002. PMC 3989204. PMID 24440755.
- ^ Cascio MJ, Jen KY (January 2018). "Cocaine/levamisole-associated autoimmune syndrome: a disease of neutrophil-mediated autoimmunity". Current Opinion in Hematology. 25 (1): 29–36. doi:10.1097/MOH.0000000000000393. PMID 29211697. S2CID 23795272.
- ^ Havakuk O, Rezkalla SH, Kloner RA (July 2017). "The Cardiovascular Effects of Cocaine". Journal of the American College of Cardiology (Review). 70 (1): 101–113. doi:10.1016/j.jacc.2017.05.014. PMID 28662796.
- ^ Fernàndez-Castillo N, Cabana-Domínguez J, Corominas R, Cormand B (January 2022). "Molecular genetics of cocaine use disorders in humans". Molecular Psychiatry. 27 (1): 624–639. doi:10.1038/s41380-021-01256-1. PMC 8960411. PMID 34453125.
- ^ Hope BT (May 1998). "Cocaine and the AP-1 transcription factor complex". Annals of the New York Academy of Sciences. 844 (1): 1–6. Bibcode:1998NYASA.844....1H. doi:10.1111/j.1749-6632.1998.tb08216.x. PMID 9668659. S2CID 11683570. Archived from the original on 28 July 2020. Retrieved 30 June 2019.
- ^ a b Kelz MB, Chen J, Carlezon WA, Whisler K, Gilden L, Beckmann AM, et al. (September 1999). "Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine". Nature. 401 (6750): 272–6. Bibcode:1999Natur.401..272K. doi:10.1038/45790. PMID 10499584. S2CID 4390717.
- ^ a b Colby CR, Whisler K, Steffen C, Nestler EJ, Self DW (March 2003). "Striatal cell type-specific overexpression of DeltaFosB enhances incentive for cocaine". The Journal of Neuroscience. 23 (6): 2488–93. doi:10.1523/JNEUROSCI.23-06-02488.2003. PMC 6742034. PMID 12657709.
- ^ a b Nestler EJ, Barrot M, Self DW (September 2001). "DeltaFosB: a sustained molecular switch for addiction". Proceedings of the National Academy of Sciences of the United States of America. 98 (20): 11042–6. Bibcode:2001PNAS...9811042N. doi:10.1073/pnas.191352698. PMC 58680. PMID 11572966.
- ^ de Souza MF, Gonçales TA, Steinmetz A, Moura DJ, Saffi J, Gomez R, et al. (April 2014). "Cocaine induces DNA damage in distinct brain areas of female rats under different hormonal conditions". Clinical and Experimental Pharmacology & Physiology. 41 (4): 265–9. doi:10.1111/1440-1681.12218. PMID 24552452. S2CID 20849951.
- ^ Alvarenga TA, Andersen ML, Ribeiro DA, Araujo P, Hirotsu C, Costa JL, et al. (January 2010). "Single exposure to cocaine or ecstasy induces DNA damage in brain and other organs of mice". Addiction Biology. 15 (1): 96–9. doi:10.1111/j.1369-1600.2009.00179.x. PMID 19878142. S2CID 21347765.
- ^ Dabin J, Fortuny A, Polo SE (June 2016). "Epigenome Maintenance in Response to DNA Damage". Molecular Cell. 62 (5): 712–27. doi:10.1016/j.molcel.2016.04.006. PMC 5476208. PMID 27259203.
- ^ Hampton WH, Hanik I, Olson IR (2019). "[Substance Abuse and White Matter: Findings, Limitations, and Future of Diffusion Tensor Imaging Research]". Drug and Alcohol Dependence. 197 (4): 288–298. doi:10.1016/j.drugalcdep.2019.02.005. PMC 6440853. PMID 30875650.
- ^ Gawin FH, Ellinwood EH (1989). "Cocaine dependence". Annual Review of Medicine. 40: 149–61. doi:10.1146/annurev.me.40.020189.001053. PMID 2658744.
- ^ Okie S (27 January 2009). "The Epidemic That Wasn't". The New York Times. Archived from the original on 6 October 2017. Retrieved 23 July 2022.
- ^ Frank DA, Augustyn M, Knight WG, Pell T, Zuckerman B (March 2001). "Growth, development, and behavior in early childhood following prenatal cocaine exposure: a systematic review". JAMA. 285 (12). Jama.ama-assn.org: 1613–1625. doi:10.1001/jama.285.12.1613. PMC 2504866. PMID 11268270.
- ^ "Cocaine Abuse and Addiction". U.S. National Institute on Drug Abuse (NIDA). Research Report Series. Archived from the original on 26 September 2007.
- ^ "Street Drugs and pregnancy". March of Dimes. Archived from the original on 5 September 2015. Retrieved 26 May 2009.
- ^ Peacock A, Tran LT, Larney S, Stockings E, Santo T, Jones H, et al. (April 2021). "All-cause and cause-specific mortality among people with regular or problematic cocaine use: a systematic review and meta-analysis". Addiction. 116 (4): 725–742. doi:10.1111/add.15239. PMC 7914269. PMID 32857457.
- ^ Barnett G, Hawks R, Resnick R (1981). "Cocaine pharmacokinetics in humans". Journal of Ethnopharmacology. 3 (2–3): 353–66. doi:10.1016/0378-8741(81)90063-5. PMID 7242115.; Jones, supra note 19; Wilkinson et al., Van Dyke et al.
- ^ Sachkova A, Jensen O, Dücker C, Ansari S, Brockmöller J (September 2022). "The mystery of the human proton-organic cation antiporter: One transport protein or many?". Pharmacology & Therapeutics. 239: 108283. doi:10.1016/j.pharmthera.2022.108283. PMID 36162727. S2CID 252527522.
- ^ Ambre J, Ruo TI, Nelson J, Belknap S (November 1988). "Urinary excretion of cocaine, benzoylecgonine, and ecgonine methyl ester in humans". Journal of Analytical Toxicology. 12 (6): 301–6. doi:10.1093/jat/12.6.301. PMID 3244269.
- ^ Kolbrich EA, Barnes AJ, Gorelick DA, Boyd SJ, Cone EJ, Huestis MA (October 2006). "Major and minor metabolites of cocaine in human plasma following controlled subcutaneous cocaine administration". Journal of Analytical Toxicology. 30 (8): 501–10. doi:10.1093/jat/30.8.501. PMID 17132243. Archived from the original on 18 July 2012.
- ^ "Schaffer Library of Drug Polocy".
- ^ Czykanski M (30 December 2015). "Cocaine Metabolites in Hair". Chemistry Views. Archived from the original on 22 July 2019. Retrieved 22 July 2019.
- ^ Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, et al. (January 2001). "Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin". Synapse. 39 (1): 32–41. doi:10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. PMID 11071707. S2CID 15573624. (Table V. on page 37)
- ^ Hummel M, Unterwald EM (April 2002). "D1 dopamine receptor: a putative neurochemical and behavioral link to cocaine action". Journal of Cellular Physiology. 191 (1): 17–27. doi:10.1002/jcp.10078. PMID 11920678. S2CID 40444893.
- ^ Carta M, Allan AM, Partridge LD, Valenzuela CF (January 2003). "Cocaine inhibits 5-HT3 receptor function in neurons from transgenic mice overexpressing the receptor". European Journal of Pharmacology. 459 (2–3): 167–9. doi:10.1016/S0014-2999(02)02867-4. PMID 12524142.
- ^ Filip M, Bubar MJ, Cunningham KA (September 2004). "Contribution of serotonin (5-hydroxytryptamine; 5-HT) 5-HT2 receptor subtypes to the hyperlocomotor effects of cocaine: acute and chronic pharmacological analyses". The Journal of Pharmacology and Experimental Therapeutics. 310 (3): 1246–54. doi:10.1124/jpet.104.068841. PMID 15131246. S2CID 25809734.
- ^ Beuming T, Kniazeff J, Bergmann ML, Shi L, Gracia L, Raniszewska K, et al. (July 2008). "The binding sites for cocaine and dopamine in the dopamine transporter overlap". Nature Neuroscience. 11 (7): 780–9. doi:10.1038/nn.2146. PMC 2692229. PMID 18568020.
- ^ Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, et al. (April 1997). "Relationship between subjective effects of cocaine and dopamine transporter occupancy". Nature. 386 (6627): 827–830. Bibcode:1997Natur.386..827V. doi:10.1038/386827a0. PMID 9126740.
- ^ Heal DJ, Gosden J, Smith SL (December 2014). "Dopamine reuptake transporter (DAT) "inverse agonism"--a novel hypothesis to explain the enigmatic pharmacology of cocaine". Neuropharmacology. CNS Stimulants. 87: 19–40. doi:10.1016/j.neuropharm.2014.06.012. PMID 24953830.
- ^ "Sigma Receptors Play Role in Cocaine-induced Suppression of Immune System". Sciencedaily.com. 6 May 2003. Archived from the original on 12 January 2011. Retrieved 9 March 2010.
- ^ Lluch J, Rodríguez-Arias M, Aguilar MA, Miñarro J (November 2005). "Role of dopamine and glutamate receptors in cocaine-induced social effects in isolated and grouped male OF1 mice". Pharmacology Biochemistry and Behavior. 82 (3): 478–87. doi:10.1016/j.pbb.2005.10.003. PMID 16313950. S2CID 13307446.
- ^ Knuepfer MM (March 2003). "Cardiovascular disorders associated with cocaine use: myths and truths". Pharmacology & Therapeutics. 97 (3): 181–222. doi:10.1016/S0163-7258(02)00329-7. PMID 12576134.
- ^ "Drugbank website "drug card", "(DB00907)" for Cocaine: Giving ten targets of the molecule in vivo, including dopamine/serotonin sodium channel affinity & K-opioid affinity". Drugbank.ca. Archived from the original on 20 February 2010. Retrieved 9 March 2010.
- ^ Uz T, Akhisaroglu M, Ahmed R, Manev H (December 2003). "The pineal gland is critical for circadian Period1 expression in the striatum and for circadian cocaine sensitization in mice". Neuropsychopharmacology. 28 (12): 2117–23. doi:10.1038/sj.npp.1300254. PMID 12865893.
- ^ McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, et al. (June 2005). "Regulation of dopaminergic transmission and cocaine reward by the Clock gene". Proceedings of the National Academy of Sciences of the United States of America. 102 (26): 9377–81. Bibcode:2005PNAS..102.9377M. doi:10.1073/pnas.0503584102. PMC 1166621. PMID 15967985.
- ^ Aguinaga D, Medrano M, Cordomí A, Jiménez-Rosés M, Angelats E, Casanovas M, et al. (February 2019). "Cocaine Blocks Effects of Hunger Hormone, Ghrelin, Via Interaction with Neuronal Sigma-1 Receptors". Molecular Neurobiology. 56 (2): 1196–1210. doi:10.1007/s12035-018-1140-7. hdl:2445/127306. PMID 29876881. S2CID 46964405.
- ^ Bouhlal S, Ellefsen KN, Sheskier MB, Singley E, Pirard S, Gorelick DA, et al. (November 2017). "Acute effects of intravenous cocaine administration on serum concentrations of ghrelin, amylin, glucagon-like peptide-1, insulin, leptin and peptide YY and relationships with cardiorespiratory and subjective responses". Drug and Alcohol Dependence. 180: 68–75. doi:10.1016/j.drugalcdep.2017.07.033. PMC 5654385. PMID 28881319.
- ^ Carey RJ, Damianopoulos EN, Shanahan AB (January 2008). "Cocaine effects on behavioral responding to a novel object placed in a familiar environment". Pharmacology Biochemistry and Behavior. 88 (3): 265–71. doi:10.1016/j.pbb.2007.08.010. PMID 17897705. S2CID 22711773.
- ^ Pillay VV (2013), Modern Medical Toxicology (4th ed.), Jaypee, pp. 553–554, ISBN 978-93-5025-965-8
- ^ "DEA fact sheet". Archived from the original on 31 October 2023. Retrieved 11 October 2023.
- ^ "Content Background: Chemical Characteristics of Cocaine". duke.edu. Archived from the original on 8 July 2024. Retrieved 4 May 2020.
- ^ Scheidweiler KB, Plessinger MA, Shojaie J, Wood RW, Kwong TC (December 2003). "Pharmacokinetics and pharmacodynamics of methylecgonidine, a crack cocaine pyrolyzate". The Journal of Pharmacology and Experimental Therapeutics. 307 (3): 1179–87. doi:10.1124/jpet.103.055434. PMID 14561847. S2CID 15619796.
- ^ Yang Y, Ke Q, Cai J, Xiao YF, Morgan JP (January 2001). "Evidence for cocaine and methylecgonidine stimulation of M(2) muscarinic receptors in cultured human embryonic lung cells". British Journal of Pharmacology. 132 (2): 451–60. doi:10.1038/sj.bjp.0703819. PMC 1572570. PMID 11159694.
- ^ Fandiño AS, Toennes SW, Kauert GF (December 2002). "Studies on hydrolytic and oxidative metabolic pathways of anhydroecgonine methyl ester (methylecgonidine) using microsomal preparations from rat organs". Chemical Research in Toxicology. 15 (12): 1543–8. doi:10.1021/tx0255828. PMID 12482236.
- ^ Lamar JV (2 June 1986). "Crack – A Cheap and Deadly Cocaine Is a Spreading Menace". Time. pp. 16–18. Archived from the original on 8 July 2024. Retrieved 17 June 2022.
- ^ Ries RK, Miller SC, Fiellin DA (2009). Principles of addiction medicine. Lippincott Williams & Wilkins. p. 137. ISBN 978-0-7817-7477-2. Archived from the original on 4 April 2014. Retrieved 5 January 2014.
- ^ Nelson G (1998). Hip Hop America. Viking Penguin. p. 40.
- ^ Embury-Dennis T. "It's legal to manufacture cocaine and heroin for medical use — and Britain is the world's biggest exporter". Business Insider. Archived from the original on 28 July 2020. Retrieved 17 March 2019.
- ^ Vera N (26 October 2021). "The Coca Story Goes Way Beyond Cola". Taste. Archived from the original on 28 May 2022. Retrieved 15 June 2022.
- ^ "Cocaine: Effects, Hazards & Warnings". Drugs.com. Archived from the original on 28 July 2020. Retrieved 17 March 2019.
- ^ Teobaldo L (1994). "The Standard Low Dose of Oral Cocaine: Used for Treatment of Cocaine Dependence" (PDF). Substance Abuse. 15 (4): 215–220. Archived (PDF) from the original on 2 June 2014.
- ^ Jenkins AJ, Llosa T, Montoya I, Cone EJ (February 1996). "Identification and quantitation of alkaloids in coca tea". Forensic Science International. 77 (3): 179–189. doi:10.1016/0379-0738(95)01860-3. PMC 2705900. PMID 8819993.
- ^ a b Humphrey AJ, O'Hagan D (October 2001). "Tropane alkaloid biosynthesis. A century-old problem unresolved". Natural Product Reports. 18 (5): 494–502. doi:10.1039/b001713m. PMID 11699882.
- ^ Dewick PM (2009). Medicinal Natural Products. Chichester: Wiley-Blackwell. ISBN 978-0-470-74276-1.
- ^ Robins RJ, Abraham TE, Parr AJ, Eagles J, Walton NJ (1997). "The Biosynthesis of Tropane Alkaloids in Datura stramonium: The Identity of the Intermediates between N-Methylpyrrolinium Salt and Tropinone". J. Am. Chem. Soc. 119 (45): 10929–10934. doi:10.1021/ja964461p.
- ^ Hoye TR, Bjorklund JA, Koltun DO, Renner MK (January 2000). "N-methylputrescine oxidation during cocaine biosynthesis: study of prochiral methylene hydrogen discrimination using the remote isotope method". Organic Letters. 2 (1): 3–5. doi:10.1021/ol990940s. PMID 10814231.
- ^ Leete E, Bjorklund JA, Couladis MM, Kim SH (1991). "Late intermediates in the biosynthesis of cocaine: 4-(1-methyl-2-pyrrolidinyl)-3-oxobutanoate and methyl ecgonine". J. Am. Chem. Soc. 113 (24): 9286–9292. doi:10.1021/ja00024a039.
- ^ Leete E, Bjorklund JA, Kim SH (1988). "The biosynthesis of the benzoyl moiety of cocaine". Phytochemistry. 27 (8): 2553–2556. Bibcode:1988PChem..27.2553L. doi:10.1016/0031-9422(88)87026-2.
- ^ Leete E, Marion L, Spenser ID (October 1954). "Biogenesis of hyoscyamine". Nature. 174 (4431): 650–1. Bibcode:1954Natur.174..650L. doi:10.1038/174650a0. PMID 13203600. S2CID 4264282.
- ^ Robins RJ, Waltons NJ, Hamill JD, Parr AJ, Rhodes MJ (October 1991). "Strategies for the genetic manipulation of alkaloid-producing pathways in plants". Planta Medica. 57 (7 Suppl): S27-35. doi:10.1055/s-2006-960226. PMID 17226220. S2CID 45912704.
- ^ Hemscheidt T, Vederas JC (2000). Leeper FJ, Vederas JC (eds.). "Tropane and Related Alkaloids". Top. Curr. Chem. Topics in Current Chemistry. 209: 175. doi:10.1007/3-540-48146-X. ISBN 978-3-540-66573-1.
- ^ Portsteffen A, Draeger B, Nahrstedt A (1992). "Two tropinone reducing enzymes from Datura stramonium transformed root cultures". Phytochemistry. 31 (4): 1135–1138. Bibcode:1992PChem..31.1135P. doi:10.1016/0031-9422(92)80247-C.
- ^ Boswell HD, Dräger B, McLauchlan WR, Portsteffen A, Robins DJ, Robins RJ, et al. (November 1999). "Specificities of the enzymes of N-alkyltropane biosynthesis in Brugmansia and Datura". Phytochemistry. 52 (5): 871–8. Bibcode:1999PChem..52..871B. doi:10.1016/S0031-9422(99)00293-9. PMID 10626376.
- ^ "Genetically modified tobacco plant produces cocaine in its leaves". New Scientist. Archived from the original on 27 November 2022. Retrieved 27 November 2022.
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 9th edition, Biomedical Publications, Seal Beach, California, 2011, pp. 390–394.
- ^ "Meet the Chemist Behind Many Popular—and Faulty—Police Drug Kits". Pacific Standard. 22 June 2016. Archived from the original on 8 August 2020. Retrieved 21 April 2020.
- ^ Gabrielson R, Sanders T (7 July 2016). "How a $2 Roadside Drug Test Sends Innocent People to Jail". The New York Times. Archived from the original on 1 January 2022. Retrieved 21 April 2020.
- ^ Travnikoff B (1 April 1983). "Semiquantitative screening test for cocaine". Analytical Chemistry. 55 (4): 795–796. doi:10.1021/ac00255a048. ISSN 0003-2700.
- ^ "Annual prevalence of use of drugs, by region and globally, 2016". World Drug Report 2018. United Nations Office on Drugs and Crime. 2018. Retrieved 7 July 2018.
- ^ a b "World Drug Report 2016 (interactive map)". United Nations Office on Drugs and Crime. 2016. Archived from the original on 9 March 2018.
- ^ "Statistical Bulletin 2018 — prevalence of drug use". www.emcdda.europa.eu. Archived from the original on 8 July 2024. Retrieved 5 February 2019.
- ^ The State of the Drugs Problem in Europe 2008 (PDF). Luxembourg: European Monitoring Centre for Drugs and Drug Addiction. 2008. pp. 58–62. Archived (PDF) from the original on 25 April 2013. Retrieved 31 December 2013.
- ^ Casciani D (4 June 2015). "Cocaine in sewage: London tops league table". BBC News. Archived from the original on 4 June 2015. Retrieved 4 June 2015.
- ^ "Cocaine & Crack". Erowid.org. Archived from the original on 6 October 2007. Retrieved 10 July 2007.
- ^ a b "Field Listing – Illicit drugs (by country)". Cia.gov. Archived from the original on 29 December 2010. Retrieved 15 January 2011.
- ^ Hesse M (2002). Alkaloids: Nature's Curse or Blessing?. Weinheim: Wiley-VCH. p. 304. ISBN 978-3-906390-24-6.
- ^ Altman AJ, Albert DM, Fournier GA (1985). "Cocaine's use in ophthalmology: our 100-year heritage". Survey of Ophthalmology. 29 (4): 300–6. doi:10.1016/0039-6257(85)90153-5. PMID 3885453.
- ^ Gay GR, Inaba DS, Sheppard CW, Newmeyer JA (1975). "Cocaine: history, epidemiology, human pharmacology, and treatment. a perspective on a new debut for an old girl". Clinical Toxicology. 8 (2): 149–78. doi:10.3109/15563657508988061. PMID 1097168.
- ^ "Drug that spans the ages: The history of cocaine". London: The Independent (UK). 2006. Archived from the original on 28 February 2010. Retrieved 30 April 2010.
- ^ Monardes N, Frampton J (1925). Joyfull Newes out of the Newe Founde Worlde. New York: Alfred Knopf.
- ^ "InterAndean Institute of Coca Sciences". www.cienciadelacoca.org. Archived from the original on 30 December 2016.
- ^ Karch SB (May 1998). A Brief History of Cocaine. Boca Raton, Fla: CRC Press. ISBN 978-0-8493-4019-2.
- ^ Luch A (3 April 2009). Molecular, Clinical and Environmental Toxicology. Basel Boston: Springer Science & Business Media. p. 20. ISBN 978-3-7643-8336-7.
- ^ Gaedcke F (1855). "Ueber das Erythroxylin, dargestellt aus den Blättern des in Südamerika cultivirten Strauches Erythroxylon Coca". Archiv der Pharmazie. 132 (2): 141–150. doi:10.1002/ardp.18551320208. S2CID 86030231. Archived from the original on 14 April 2021. Retrieved 3 September 2020.
- ^ a b Niemann A (1860). "Ueber eine neue organische Base in den Cocablättern". Archiv der Pharmazie. 153 (2 and 3): 129–155, 291–308. doi:10.1002/ardp.18601530202. S2CID 98195820. Archived from the original on 28 July 2020. Retrieved 30 June 2019.
- ^ Harper D. "Cocaine". Online Etymology Dictionary.
- ^ Singh S (March 2000). "Chemistry, design, and structure-activity relationship of cocaine antagonists" (PDF). Chemical Reviews. 100 (3): 925–1024. doi:10.1021/cr9700538. PMID 11749256. Archived (PDF) from the original on 4 March 2016.
Page 970 (46th page of article) first, ninth, and tenth lines
- ^ (a) Willstatter R (1903). "Synthese der Ecgoninsäure" [Synthesis of Tropine]. Liebigs Ann. (in German). 326 (1–2): 23. doi:10.1002/jlac.19033260105. (b) Robinson RJ (1917). "LXIII. A synthesis of tropinone". J. Chem. Soc., Trans. 111: 762–768. doi:10.1039/CT9171100762. Archived from the original on 30 September 2020. Retrieved 30 June 2019. (c) Schopf C, Lehman G (1935). "Die Synthese des Tropinons, Pseudopelletierins, Lobelanins und verwandter Alkaloide unter physiologischen Bedingungen" [The synthesis of tropinone, pseudopelletierin, lobelanin and related alkaloids under physiological conditions]. Liebigs Ann. (in German). 518: 1–37. doi:10.1002/jlac.19355180102.
- ^ Yentis SM, Vlassakov KV (March 1999). "Vassily von Anrep, forgotten pioneer of regional anesthesia". Anesthesiology. 90 (3): 890–5. doi:10.1097/00000542-199903000-00033. PMID 10078692.
- ^ Halsted W (1885). "Practical comments on the use and abuse of cocaine". New York Medical Journal. 42: 294–295.
- ^ Corning JL (1885). "An experimental study". New York Medical Journal. 42: 483.
- ^ "Experience Vin Mariani today | Grupo Mariani S.A". Cocanaturally.com. Archived from the original on 8 February 2011. Retrieved 15 January 2011.
- ^ Freud S (1975). Byck R (ed.). Cocaine Papers. Stonehill. ISBN 0-88373-010-3.
- ^ "How a Young Sigmund Freud Researched & Got Addicted to Cocaine, the New "Miracle Drug," in 1894". Open Culture. Archived from the original on 7 March 2017.
- ^ "Sigmund Freud and Cocaine". cocaine.org. Archived from the original on 19 January 2017.
- ^ Musto DF (1989). "America's First Cocaine Epidemic". The Wilson Quarterly. 13 (3): 59–64. JSTOR 40257908. PMID 11619697. Archived from the original on 12 July 2022. Retrieved 12 July 2022.
- ^ Barlow W (1989). Looking Up At Down: The Emergence of Blues Culture. Philadelphia: Temple University Press. p. 207. ISBN 0-87722-583-4.
- ^ Streatfeild D (2003). Cocaine: An Unauthorized Biography. Picador. ISBN 978-0-312-42226-4.
- ^ White T (14 August 1993). "Catchin' Cab: The Magic of Calloway". Billboard. p. 3. Archived from the original on 8 July 2024. Retrieved 13 January 2022.
- ^ The Big Broadcast (1932) (Full Movie). 8 January 2021. Event occurs at 1:20:29. Archived from the original on 13 January 2022. Retrieved 13 January 2022 – via YouTube.
- ^ "Jeevan Vasagar: cocaine-based "wonder drug" tested on concentration camp inmates". Amphetamines.com. 19 November 2002. Archived from the original on 27 February 2011. Retrieved 15 January 2011.
- ^ a b Ryzik M (10 June 2007). "Cocaine: Hidden in Plain Sight". The New York Times. Archived from the original on 11 August 2017. Retrieved 18 May 2017.
- ^ "The Buyers – A Social History of America's Most Popular Drugs". FRONTLINE. PBS. Archived from the original on 14 May 2017. Retrieved 18 May 2017.
- ^ Brisbane FL, Womble M (1985). Treatment of Black Alcoholics. Psychology Press. ISBN 978-0-86656-403-8. Archived from the original on 10 September 2017. Retrieved 18 May 2017.
- ^ Waldorf D, Reinarman C, Murphy S (June 1992). Cocaine Changes: The Experience of Using and Quitting. Temple University Press. p. 95. ISBN 978-1-56639-013-2. Retrieved 18 May 2017.
- ^ a b Current JD. "Cocaine". Pharmacology for Anesthetists. p. 27.
- ^ Kozel NJ, Adams EH (July 1996). Cocaine Use in America. DIANE Publishing. ISBN 978-0-7881-2968-1.
- ^ Spillane JF (11 January 2000). Cocaine. Baltimore, MD: JHU Press. ISBN 978-0-8018-6230-4.
- ^ Kilmer B (2017). "Mixed messages: Is cocaine consumption in the U.S. going up or down?". Brookings. Archived from the original on 29 June 2024. Retrieved 29 June 2024.
- ^ Great Britain: Parliament: House of Commons: Home Affairs Committee (3 March 2010). The cocaine trade. The Stationery Office. p. 22. ISBN 978-0-215-54425-4.
- ^ "Apple Sanity – Fetish – Blow: War on Drugs VS. Cocaine". Applesanity.com. 17 June 2008. Archived from the original on 17 June 2008. Retrieved 13 November 2011.
- ^ "Cocaine Market". Havocscope.com. 28 April 2008. Archived from the original on 11 November 2012. Retrieved 9 March 2010.
- ^ WHO/UNICRI (4 February 2010). "The WHO Cocaine Project". Transnational Institute. Archived from the original on 9 August 2012. Retrieved 8 June 2012.
- ^ "Cocaine use doubles in a decade". Sydney Morning Herald. 15 October 2010. Archived from the original on 18 October 2010. Retrieved 19 October 2010.
- ^ Ribeiro M, Trevizol AP, Frajzinger R, Ribeiro A, Speierl H, Pires L, et al. (July 2019). "Adulterants in crack cocaine in Brazil". Trends in Psychiatry and Psychotherapy. 41 (2): 186–190. doi:10.1590/2237-6089-2017-0143. PMID 31314858.
- ^ United Nations Office on Drugs and Crime. Laboratory and Scientific Section (2005). Methods for Impurity Profiling of Heroin and Cocaine. United Nations Publications. ISBN 978-92-1-148206-5.
- ^ EMCDDA (2007). "EMCDDA Retail Cocaine Purity Study". Archived from the original on 1 January 2014. Retrieved 31 December 2013.
- ^ Franklin J (18 August 2009). "The world's first cocaine bar". The Guardian. ISSN 0261-3077. Archived from the original on 12 January 2017. Retrieved 23 December 2016.
- ^ Grisaffi T. "The Cato Accord: Bolivia's Humane and Effective Approach to Controlling Coca Cultivation" (PDF). ain-bolivia.org. Archived (PDF) from the original on 3 May 2016. Retrieved 12 January 2017.
- ^ "Therapeutic Goods (Poisons Standard—October 2023) Instrument 2023". Federal Register of Legislation. Australian Government. 26 September 2023. Retrieved 22 January 2024.
- ^ "Illicit drug use". Australian Institute of Health and Welfare. 13 December 2023. Archived from the original on 20 January 2024. Retrieved 22 January 2024.
- ^ "Misuse of Drugs Act 1981" (PDF). Western Australian Legislation. Government of Western Australia Department of Justice Parliamentary Counsel's Office. Archived (PDF) from the original on 17 February 2024. Retrieved 22 January 2024.
- ^ Gootenberg 1999, p. 37.
- ^ Madge 2001, p. 106.
- ^ "Narcotic drug addiction". American Medicine. American-Medicine Publishing Company: 799. November 1915. Archived from the original on 9 May 2018. Retrieved 29 April 2018.
- ^ a b c Gootenberg 1999, p. 40.
- ^ Brown E, Barganier G (2018). Race and Crime: Geographies of Injustice. Oakland, California: University of California Press. pp. 207–209. ISBN 978-0-520-29418-9. Archived from the original on 12 April 2023. Retrieved 21 November 2021.
- ^ Moore NM (2015). The Political Roots of Racial Tracking in American Criminal Justice. New York, NY: Cambridge University Press. p. 270. ISBN 978-1-107-02297-3. Archived from the original on 6 April 2023. Retrieved 21 November 2021.
- ^ Glaser J (2015). Suspect Race: Causes and Consequences of Racial Profiling. New York, NY: Oxford University Press. p. 7. ISBN 978-0-19-537040-9. Archived from the original on 12 April 2023. Retrieved 21 November 2021.
- ^ "Cocaine: Seizures, 1998–2003" (PDF). World Drug Report 2006. Vol. 2. New York: United Nations. 2006. Archived (PDF) from the original on 14 June 2007.
- ^ "Colombia". CIA World Factbook. Archived from the original on 18 June 2021. Retrieved 24 January 2021.
- ^ "Peru Overtakes Colombia as Top Cocaine Producer". NBC News. 31 July 2012. Archived from the original on 4 March 2016.
- ^ Ciro E, Ryder M, Sánchez S (2024). "Peace and reparations in legal drug markets in Colombia". Futures. 157: 103336. doi:10.1016/j.futures.2024.103336.
- ^ Streatfeild D (2003). Cocaine: An Unauthorized Biography. Macmillan. ISBN 978-0-312-42226-4. Archived from the original on 15 January 2014. Retrieved 5 January 2014.
- ^ Messina JP, Delamater PL (10 January 2006). "Defoliation and the war on drugs in Putumayo, Colombia". International Journal of Remote Sensing. 27 (1): 121–128. Bibcode:2006IJRS...27..121M. doi:10.1080/01431160500293708. ISSN 0143-1161.
- ^ Ferreira JF, Smeda RJ, Duke SO (1997). "Control of coca plants ( Erythroxylum coca and E. novogranatense ) with glyphosate". Weed Science. 45 (4): 551–556. doi:10.1017/S0043174500088809. ISSN 0043-1745.
- ^ Marcela I (August 2010). "Who crops coca and why? The case of Colombian farmers" (PDF). Econstor. Archived (PDF) from the original on 28 April 2024. Retrieved 28 April 2024.
- ^ "Colombia: 'I'm not proud cultivating coca, but we have no choice'". Al Jazeera. Archived from the original on 8 July 2024. Retrieved 28 April 2024.
- ^ "National Drug Threat Assessment 2006". National Drug Intelligence Center. 2006. Archived from the original on 11 November 2010.
- ^ "Cocaine Seized Worldwide Highest Ever in 2011". Flare Network (Flarenetwork.org). 18 January 2012. Archived from the original on 6 January 2014. Retrieved 5 January 2014.
- ^ "Colombia". U.S. Department of State. State.gov. Archived from the original on 22 January 2017. Retrieved 26 March 2013.
- ^ Amara SB, Koslowski T, Zaidi A (2021). "Quantum Chemistry of Cocaine and its Isomers I: Energetics, Reactivity and Solvation". South African Journal of Chemistry. 75. doi:10.17159/0379-4350/2021/v75a3.
- ^ Drake LR, Scott PJ (October 2018). "DARK Classics in Chemical Neuroscience: Cocaine". ACS Chemical Neuroscience. 9 (10): 2358–2372. doi:10.1021/acschemneuro.8b00117. PMC 6197930. PMID 29630337.
- ^ "How Much Is a Gram of Coke?". New Health Advisor. 3 September 2015. Archived from the original on 7 March 2017.
- ^ Jacobson R (2006). Illegal drugs: America's anguish (2005 ed.). Farmington Hills, Michigan: Thomson Gale. ISBN 978-1-4144-0419-6.
- ^ "2015 National Drug Threat Assessment Summary" (PDF). Drug Enforcement Administration. United States Department of Justice: Drug Enforcement Administration. October 2015. pp. 1–2. Archived from the original (PDF) on 10 April 2016. Retrieved 10 April 2016.
Mexican TCOs pose the greatest criminal drug threat to the United States; no other group is currently positioned to challenge them. These Mexican poly-drug organizations traffic heroin, methamphetamine, cocaine, and marijuana throughout the United States, using established transportation routes and distribution networks. ... While all of these Mexican TCOs transport wholesale quantities of illicit drugs into the United States, the Sinaloa Cartel appears to be the most active supplier. The Sinaloa Cartel leverages its expansive resources and dominance in Mexico to facilitate the smuggling and transportation of drugs throughout the United States.
- ^ "Cocaine. Puerto Rico and the U.S. Virgin Islands Drug Threat Assessment". National Drug Intelligence Center. 2003. Archived from the original on 29 June 2024. Retrieved 29 June 2024.
- ^ Zimmerman S (23 October 2012). A History of Smuggling in Florida. Arcadia Publishing. ISBN 978-1-61423-356-5.
- ^ Corben B, Spellman A (May 2009). Cocaine Cowboys. powerHouse Books. ISBN 978-1-57687-503-2.
- ^ United Nations Office on Drugs and Crime (16 December 2015). World Drug Report 2015. United Nations. p. XV. ISBN 978-92-1-057300-9.
- ^ a b Fleetwood J (18 June 2014). Drug Mules. Springer. ISBN 978-1-137-27190-7.
- ^ "Drug mules: Swallowed by the illicit drug trade". UN Office of Drugs and Crime. Archived from the original on 8 July 2024. Retrieved 29 June 2024.
- ^ Klein A, Day M, Harriott A (18 July 2013). Caribbean Drugs. Zed Books Ltd. ISBN 978-1-84813-622-9.
- ^ Sutton H (August 2020). "3 Types Of Go-Fast Narco Boats The Coast Guard Faces". Forbes. Archived from the original on 6 February 2024. Retrieved 29 June 2024.
- ^ "Coast Guard hunts drug-running semi-subs". CNN. 20 March 2008. Archived from the original on 21 March 2008. Retrieved 20 March 2008.
- ^ "Meth Info". Methproject.org. Archived from the original on 27 March 2010.
- ^ "Drugs of Abuse". City of Denison Iowa. Archived from the original on 6 November 2011. Retrieved 13 November 2011.
- ^ "Drugs: Pricing Power". The Economist. 28 June 2007. Archived from the original on 6 January 2014.
Prices: USA around $110/g, Israel/Germany/Britain around $46/g, Colombia $2/g, New Zealand recordbreaking $714.30/g.
- ^ European Monitoring Centre for Drugs and Drug Addiction (2008). Annual report: the state of the drugs problem in Europe (PDF). Luxembourg: Office for Official Publications of the European Communities. p. 59. ISBN 978-92-9168-324-6. Archived (PDF) from the original on 25 April 2013. Retrieved 31 December 2013.
- ^ The Cocaine Threat: A Hemispheric Perspective (PDF). United States Department of Defense. Archived from the original (PDF) on 11 September 2008.
- ^ United Nations (June 2010). World Drug Report 2010. United Nations Publications. p. 77. ISBN 978-92-1-148256-0. Archived from the original on 26 April 2016.
General and cited references
- Gootenberg P, ed. (1999). Cocaine: Global Histories. London: Routledge. ISBN 978-0-203-02646-5.
- Madge T (2001). White Mischief: A Cultural History of Cocaine. Edinburgh: Mainstream Publishing Company. ISBN 978-1-84018-405-1.
- Spillane JF, ed. (2000). Cocaine: From Medical Marvel to Modern Menace in the United States, 1884–1920. Baltimore and London: The Johns Hopkins University Press. ISBN 978-0-8018-6230-4.
Further reading
- Feiling T (2009). The Candy Machine: How Cocaine Took Over the World. London: Penguin. ISBN 978-0-14-103446-1.
External links
- "Cocaine". European Monitoring Centre for Drugs.
- Cocaine
- 1855 introductions
- 1855 in science
- Alkaloids found in Erythroxylum
- Anorectics
- Benzoate esters
- Carboxylate esters
- Cardiac stimulants
- CYP2D6 inhibitors
- Euphoriants
- German inventions
- Glycine receptor agonists
- Local anesthetics
- Methyl esters
- Otologicals
- Powders
- Secondary metabolites
- Serotonin–norepinephrine–dopamine reuptake inhibitors
- Sigma agonists
- Stimulants
- Sympathomimetic amines
- Teratogens
- Tropane alkaloids found in Erythroxylum coca
- Vasoconstrictors
- Obsolete medications


![Delphic analysis regarding 20 popular recreational drugs based on expert opinion in the UK. Cocaine was ranked the 2nd in dependence and physical harm and 3rd in social harm.[67]](http://upload.wikimedia.org/wikipedia/commons/thumb/7/73/Rational_harm_assessment_of_drugs_bar_plot.svg/504px-Rational_harm_assessment_of_drugs_bar_plot.svg.png)